Entinostat
 |
|
| Names | |
|---|---|
|
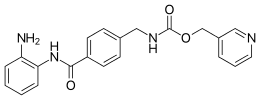
IUPAC name
Pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]
|
|
| Other names
SNDX-275; MS-275
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.158.999 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C21H20N4O3 | |
| Molar mass | 376.4085 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Entinostat, also known as SNDX-275 and MS-275, is a benzamide histone deacetylase inhibitor undergoing clinical trials for treatment of various cancers.
Entinostat inhibits class I HDAC1 and HDAC3 with IC50 of 0.51 μM and 1.7 μM, respectively.
Syndax pharmaceuticals currently holds the rights to Entinostat and recently received $26.6 million in funds to advance treatments of resistant cancers using epigenetic tools.
There is an ongoing phase II trial studying the effect of entinostat on Hodgkin's lymphoma. It is in other phase II trials for advanced breast cancer (in combination with aromatase inhibitors) and for metastatic lung cancer (in combination with erlotinib). As of September 2013, the Food and Drug Administration is working with the industry to design phase III clinical trials. They seek to evaluate the application of Entinostat for the reduction, or prevention of, treatment resistance to aromatase inhibitors in hormone receptor positive breast cancer.
...
Wikipedia
