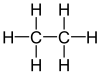
ETHANE
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Ethane
|
|||
|
Systematic IUPAC name
Dicarbane (never recommended)
|
|||
| Identifiers | |||
|
74-84-0 |
|||
| 3D model (Jmol) | Interactive image | ||
| 1730716 | |||
| ChEBI |
CHEBI:42266 |
||
| ChEMBL |
ChEMBL135626 |
||
| ChemSpider |
6084 |
||
| ECHA InfoCard | 100.000.741 | ||
| EC Number | 200-814-8 | ||
| 212 | |||
| MeSH | Ethane | ||
| PubChem | 6324 | ||
| RTECS number | KH3800000 | ||
| UNII |
L99N5N533T |
||
| UN number | 1035 | ||
|
|||
|
|||
| Properties | |||
| C2H6 | |||
| Molar mass | 30.07 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density |
|
||
| Melting point | −182.8 °C; −296.9 °F; 90.4 K | ||
| Boiling point | −88.5 °C; −127.4 °F; 184.6 K | ||
| 56.8 mg L−1 | |||
| Vapor pressure | 3.8453 MPa (at 21.1 °C) | ||
|
Henry's law
constant (kH) |
19 nmol Pa−1 kg−1 | ||
| Acidity (pKa) | 50 | ||
| Basicity (pKb) | -36 | ||
| -37.37·10−6 cm3/mol | |||
| Thermochemistry | |||
| 52.49 J K−1 mol−1 | |||
|
Std enthalpy of
formation (ΔfH |
−84 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−1561.0–−1560.4 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet |
See: data page inchem.org |
||
| GHS pictograms |  |
||
| GHS signal word | DANGER | ||
| H220, H280 | |||
| P210, P410+403 | |||
|
EU classification (DSD)
|
|||
| R-phrases | R12 | ||
| S-phrases | (S2), S9, S16, S33 | ||
| NFPA 704 | |||
| Flash point | −135 °C (−211 °F; 138 K) | ||
| 472 °C (882 °F; 745 K) | |||
| Explosive limits | 2.9–13% | ||
| Related compounds | |||
|
Related alkanes
|
|||
|
Related compounds
|
|||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Ethane (/ˈɛθeɪn/ or /ˈiːθeɪn/) is an organic chemical compound with chemical formula C2H6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical byproduct of petroleum refining. Its chief use is as for ethylene production.
Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages.
Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile (ethyl cyanide) and ethyl iodide with potassium metal, and, as did Faraday, by the electrolysis of aqueous acetates. They, however, mistook the product of these reactions for methyl radical rather than the dimer of methyl, ethane. This error was corrected in 1864 by Carl Schorlemmer, who showed that the product of all these reactions was in fact ethane.
...
Wikipedia