Duloxetine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Cymbalta, many others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604030 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~ 50% (32% to 80%) |
| Protein binding | ~ 95% |
| Metabolism | Liver, two P450 isozymes, CYP2D6 and CYP1A2 |
| Biological half-life | 12.1 hours |
| Excretion | 70% in urine, 20% in feces |
| Identifiers | |
|
|
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.129.057 |
| Chemical and physical data | |
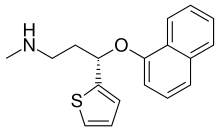
| Formula | C18H19NOS |
| Molar mass | 297.41456 g/mol |
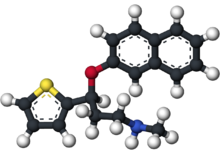
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Duloxetine, sold under the brand name Cymbalta among others, is mostly prescribed for major depressive disorder, generalized anxiety disorder, fibromyalgia and neuropathic pain.
It is a thiophene derivative and a selective neurotransmitter reuptake inhibitor for serotonin, norepinephrine, and to a lesser degree dopamine. It belongs to a class of heterocyclic antidepressants known as a serotonin–norepinephrine reuptake inhibitors (SNRI).
Duloxetine failed to receive US approval for stress urinary incontinence amid concerns over liver toxicity and suicidal events; however, it was approved for this indication in the UK, where it is recommended as an add-on medication in stress urinary incontinence instead of surgery. It was originally made by Eli Lilly.
The main uses of duloxetine are in major depressive disorder, generalized anxiety disorder, urinary incontinence, neuropathic pain, chronic musculoskeletal pain, and fibromyalgia.
Duloxetine is recommended as a first line agent for the treatment of chemotherapy-induced neuropathy by the American Society of Clinical Oncology, as a first-line therapy for fibromyalgia in the presence of mood disorders by the German Interdisciplinary Association for Pain Therapy, as a Grade B recommendation for the treatment of diabetic neuropathy by the American Association for Neurology and as a level A recommendation in certain neuropathic states by the European Federation of Neurological Societies.
...
Wikipedia
