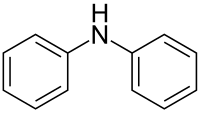
Diphenylamine
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
N-Phenylaniline
|
|
| Other names
(Diphenyl)amine
Diphenylamine (deprecated) Diphenylazane N-Phenylbenzenamine Anilinobenzene (Phenylamino)benzene N,N-Diphenylamine C.I. 10355 Phenylbenzenamine |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| Abbreviations | DPA |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.128 |
| KEGG | |
| RTECS number | 9 |
| UNII | |
|
|
|
|
| Properties | |
| C12H11N | |
| Molar mass | 169.23 g/mol |
| Appearance | White, tan, amber, or brown crystals |
| Odor | pleasant, floral |
| Density | 1.2 g/cm3 |
| Melting point | 53 °C (127 °F; 326 K) |
| Boiling point | 302 °C (576 °F; 575 K) |
| 0.03% | |
| Vapor pressure | 1 mmHg (108°C) |
| Acidity (pKa) | 0.79 |
| -109.7·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Toxic. Possible mutagen. Possible teratogen. Harmful in contact with skin, and if swallowed or inhaled. Irritant. |
| Safety data sheet | See: data page |
| R-phrases | R23 R24 R25 R33 R50 R53 |
| S-phrases | S36 S37 S45 S60 S61 |
| NFPA 704 | |
| Flash point | 152 °C (306 °F; 425 K) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
none |
|
REL (Recommended)
|
TWA 10 mg/m3 |
|
IDLH (Immediate danger)
|
N.D. |
| Related compounds | |
|
Related Amine
|
Aniline |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized impurities. Diphenylamine dissolves well in many common organic solvents, and is moderately soluble in water. It is used mainly for its antioxidant properties.
Diphenylamine is manufactured by the thermal deamination of aniline over oxide catalysts:
It is a weak base, with a Kb of 10−14. With strong acids, it forms salts. For example, treatment with sulfuric acid gives the bisulfate [(C6H5)2NH2]+[HSO4]− as a white or yellowish powder with m.p. 123-125 °C.
Diphenylamine undergoes various cyclisation reactions. With sulfur, it gives phenothiazine, a precursor to pharmaceuticals.
With iodine, it undergoes dehydrogenation to give carbazole, with release of hydrogen iodide:
Arylation with iodobenzene gives triphenylamine.
Diphenylamine is used as a pre- or postharvest scald inhibitor for apples applied as an indoor drench treatment. Its anti-scald activity is the result of its antioxidant properties, which protect the apple skin from the oxidation products of alpha-farnesene during storage. Scald (or "Apple scald") is physical injury that manifests in brown spots after fruit is removed from cold storage.
Alkylated diphenylamines function as antioxidants in lubricants, approved for use in machines, in which contact with food is not ruled out.
Diphenylamine derivatives, such as ring-alkylated derivatives of diphenylamine are used as anti-ozonants in the manufacture of rubber products, reflecting the antioxidant nature of aniline derivatives.
...
Wikipedia

