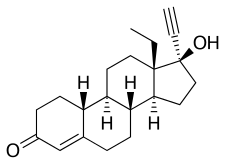
Dextronorgestrel
 |
|

|
|
| Clinical data | |
|---|---|
| Trade names | Ovral, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a602008 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 5–14 hours |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ECHA InfoCard | 100.026.758 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.45 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Norgestrel, sold under the brand name Ovral among others, is a progestin that is used in combination with an estrogen in hormonal contraceptives. It has also been used as an emergency contraceptive in the Yuzpe regimen.
Norgestrel, also known as rac-13-ethyl-17α-ethynyl-19-nortestosterone or as rac-13-ethyl-17α-ethynylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone. It is a racemic mixture of stereoisomers dextronorgestrel (the C13α isomer) and levonorgestrel (the C13β isomer), the former of which is inactive (making norgestrel exactly half as potent as levonorgestrel). Norgestrel is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.
Norgestrel was first introduced, as a contraceptive in combination with ethinylestradiol under the brand name Ovrette in the United States, in 1968, and was subsequently marketed in many other countries.
Norgestrel is the generic name of the drug and its INN, USAN, USP, BAN, DCF, DCIT, and JAN.
...
Wikipedia
