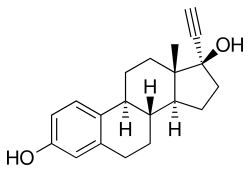
Ethinylestradiol
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛθᵻnᵻlˌiːstrəˈdaɪ.əl/ |
| Trade names | Estinyl, others |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a604032 |
| Pregnancy category |
|
| Routes of administration |
• By mouth (tablet) • Transdermal (patch) • Vaginal (ring) |
| ATC code | G03CA01 (WHO) L02AA03 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 38–48% |
| Protein binding | 97–98% (to albumin; does not bind to SHBG) |
| Metabolism | Liver (primarily CYP3A4) |
| Biological half-life | 7–36 hours |
| Excretion | Feces: 62% Urine: 38% |
| Identifiers | |
|
|
| Synonyms | 17α-Ethynylestradiol; 17α-Ethynylestra-1,3,5(10)-triene-3,17β-diol; NSC-10973 |
| CAS Number |
57-63-6 |
| PubChem (CID) | 5991 |
| IUPHAR/BPS | 7071 |
| DrugBank |
DB00977 |
| ChemSpider |
5770 |
| UNII |
423D2T571U |
| KEGG |
D00554 |
| ChEBI |
CHEBI:4903 |
| ChEMBL |
CHEMBL691 |
| ECHA InfoCard | 100.000.311 |
| Chemical and physical data | |
| Formula | C20H24O2 |
| Molar mass | 296.403 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Ethinylestradiol (EE) is a synthetic, steroidal estrogen and a derivative of estradiol, the major endogenous estrogen in humans. It is an estrogen that is active when taken by mouth and is used in almost all formulations of combined birth control pills, and is nearly the exclusive estrogen used for this purpose.
EE was patented in 1935 and came into medical use in 1943. It started being used in COCs in 1964.
As Estinyl, EE was formerly used for hormone replacement therapy in menopause and the treatment of female hypogonadism, loss of menstruation, dysmenorrhea, acne, prostate cancer, and breast cancer. However, in more recent times, EE is mainly used in COCs. In contraception, due to concerns of unopposed estrogen action and the possible increased risk of endometrial cancer that accompanies this, EE is formulated in combination with progestins. EE is little used in menopausal hormone replacement therapy.
EE has been used at very high dosages (1–2 mg/day) in the treatment of prostate cancer.
EE should be avoided in women with a history of or known susceptibility to thrombosis (blood clots), particularly venous thromboembolism (VTE).
Due to risk of cholestatic hepatotoxicity, it is widely considered that COCs containing EE should be avoided in women with a history of cholestasis of pregnancy, hepatic tumors, active hepatitis, and familial defects in biliary excretion.
...
Wikipedia
