Decaborane
 |
|
| Names | |
|---|---|
| Other names
decaborane
decaboron tetradecahydride |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.904 |
| EC Number | 241-711-8 |
|
|
|
|
| Properties | |
| Molar mass | 122.22 g/mol |
| Appearance | White crystals |
| Odor | bitter, chocolate-like |
| Density | 0.94 g/cm3 |
| Melting point | 97–98 °C (207–208 °F; 370–371 K) |
| Boiling point | 213 °C (415 °F; 486 K) |
| Solubility in other solvents | Slightly, in cold water. [1] |
| Vapor pressure | 0.2 mmHg |
| Hazards | |
| Main hazards | may ignite spontaneously on exposure to air |
| Flash point | 80 °C; 176 °F; 353 K |
| 149 °C (300 °F; 422 K) | |
| Lethal dose or concentration (LD, LC): | |
|
LC50 (median concentration)
|
276 mg/m3 (rat, 4 hr) 72 mg/m3 (mouse, 4 hr) 144 mg/m3 (mouse, 4 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 0.3 mg/m3 (0.05 ppm) [skin] |
|
REL (Recommended)
|
TWA 0.3 mg/m3 (0.05 ppm) ST 0.9 mg/m3 (0.15 ppm) [skin] |
|
IDLH (Immediate danger)
|
15 mg/m3 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Decaborane, also called decaborane(14), is the borane with the chemical formula B10H14. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, with a chocolate-like odor.
B10H14 possesses a strong characteristic odor, sometimes described as musty or intensely bitter, chocolate-like that is unique to boranes. The physical characteristics of decaborane(14) resemble those of the organic compounds, such as naphthalene and anthracene, in that it can be sublimed under vacuum at moderate temperatures. Sublimation is the common method of purification. Like organic compounds, decaborane is highly flammable, but, like other boron hydrides, it burns with a bright green flame. It is not sensitive to moist air, although it hydrolyzes in boiling water, releasing hydrogen and giving a solution of boric acid. It is soluble in cold water as well as a variety of non-polar and moderately polar solvents.
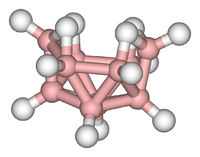
In decaborane, the B10 framework resembles an incomplete octadecahedron. Each boron has one "radial" hydride, and four boron atoms near the open part of the cluster feature extra hydrides. In the language of cluster chemistry, the structure is classified as "nido".
It is commonly synthesized via the pyrolysis of smaller boron hydride clusters. For example, pyrolysis B2H6 or B5H9 gives decaborane, with loss of H2. On a laboratory scale, sodium borohydride is treated with boron trifluoride to give NaB11H14, which is acidified to release borane and hydrogen gas.
It reacts with Lewis bases (L) such as CH3CN and Me2S, to form derivatives with the formula B10H12·2L.
...
Wikipedia
