Dauricine
 |
|
| Names | |
|---|---|
|
IUPAC name
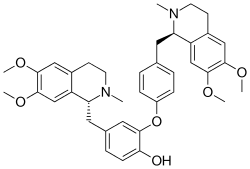
4-{[(1R)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl}-2-(4-{[(1R)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl}phenoxy)phenol
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.208.622 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C38H44N2O6 | |
| Molar mass | 624.76576 |
| Density | 1.186 g/mL |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Dauricine is a plant metabolite, chemically classified as a phenol, an aromatic ether, and an isoquinoline alkaloid. It has been isolated from the Asian vine Menispermum dauricum, commonly known as Asian moonseed, and the North American vine Menispermum canadense, commonly known as Canadian moonseed. Scientists Tetsuji Kametani and Keiichiro Fukumoto of Japan are credited with being the first to synthesize dauricine in 1964, using both the Arndt-Eistert reaction and Bischler-Napieralski reaction to do so. Dauricine has been show to play a variety of biological roles in the human body, from inhibiting cancer cell growth to blocking cardiac transmembrane Na+, K+, and Ca2+ ion currents.
Dauricine and Colon Cancer:
Dauricine has been shown to have anti-tumor effects in colon cancer. There is evidence to suggest that dauricine suppresses colon cancer cell growth and invasion in a dose-dependent manner. Dauricine has also been shown to induce apoptosis in colon cancer cells by facilitating the cleavage of caspase3 and poly ADP ribose polymerase (PARP). Furthermore, it is thought that dauricine suppresses tumor necrosis factor-induced (TNF-induced) nuclear factor-kappa B (NF-κB) activation by inhibiting the p65 nuclear translocation in colon cancer cells. Together, these discoveries help scientists better understand the link between dauricine and the suppression of colon cancer tumor cells.
Dauricine and Lung Cancer:
Dauricine has been shown to cause cytotoxicity in human lung cell lines, including BEAS-2B, WI-38, and A5449, which have been implicated in lung bronchus, lung fibroblast, and lung cancer, respectively. One study found that after 24-hour exposure to 40 µM dauricine, there was more than 60% cell death in the aforementioned human lung cell lines. Moreover, CYP3A, a class of human recombinant P450 enzymes, have been found to activate dauricine in human lung cells, resulting in the formation of quinone methide metabolite whose role in lung cytotoxicity needs to be further studied. Overall, dauricine-induced cytotoxicity in human lung cells raises concerns about the use of dauricine and its analogues in pharmaceuticals.
...
Wikipedia
