DBU (chemistry)
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
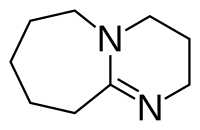
2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine
|
|
| Other names
DBU,Diazabicycloundecene
|
|
| Identifiers | |
|
6674-22-2 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
73246 |
| ECHA InfoCard | 100.027.013 |
|
|
|
|
| Properties | |
| C9H16N2 | |
| Molar mass | 152.24 g/mol |
| Appearance | Colorless liquid |
| Density | 1.018 g/mL liquid |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 80 to 83 °C (176 to 181 °F; 353 to 356 K) (0.6 mmHg); 261 °C (1 atm) |
| Acidity (pKa) | 13.5±1.5 (pKa of conjugate acid in water); 24.34 (pKa of conjugate acid in acetonitrile) |
| Hazards | |
| R-phrases | R22 R34 |
| S-phrases | S24 S25 |
| Flash point | 119.9 °C (247.8 °F; 393.0 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base.
Although DBU is typically produced synthetically, it is also an alkaloid isolated from the sponge Niphates digitalis. The biosynthesis of DBU has been proposed to begin with suberic aldehyde and diaminopropane.
As a reagent in organic chemistry, DBU is used as a catalyst, a complexing ligand, and a non-nucleophilic base. It is also used as a curing agent for epoxy. It is used in fullerene purification with trimethylbenzene (it reacts with C70 and higher fullerenes, but not to C60 fullerenes); and it is also used as a catalyst in polyurethane production. It has a strong catalyst effect for the reactions of alicyclic and aliphatic isocyanates. It also exhibited its dual character (base and nucleophile) in the synthesis of aryl- & styryl- terminal acetylenes.
...
Wikipedia
