Copper(II) fluoride
 |
|
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Copper difluoride
|
|
| Other names
Cupric fluoride; Copper fluoride; Copper (2+) Difluoride
|
|
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.225 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| CuF2 | |
| Molar mass | 101.543 g/mol (anhydrous) 137.573 g/mol (dihydrate) |
| Appearance | White crystalline powder When hydrated: Blue |
| Density | 4.23 g/cm3 (anhydrous) 2.934 g/cm3 (dihydrate) |
| Melting point | 836 °C (1,537 °F; 1,109 K) (anhydrous) 130 °C (dihydrate, decomposes) |
| Boiling point | 1,676 °C (3,049 °F; 1,949 K) (anhydrous) |
| Solubility in other solvents | Hygroscopic |
| +1050.0·10−6 cm3/mol | |
| Hazards | |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu) |
|
REL (Recommended)
|
TWA 1 mg/m3 (as Cu) |
|
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu) |
| Related compounds | |
|
Other anions
|
Copper(II) bromide Copper(II) chloride |
|
Other cations
|
Silver(II) fluoride Cobalt(II) fluoride |
|
Related compounds
|
Copper(I) fluoride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
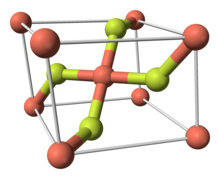
Copper(II) fluoride is an inorganic compound with the chemical formula CuF2. It is a white or green, crystalline, hygroscopic solid. It has a rutile-type crystal structure similar to other fluorides of chemical formulae MF2.
Copper(II) fluoride is made of a crystalline structure. It has a monoclinic crystal structure and can not achieve a higher-symmetry structure. It forms rectangular prisms and has parallelogram type base. It contains three vectors where one vector is perpendicular to two parallel vectors in the structure.
It has been shown that aromatic hydrocarbons react with copper(II) fluoride, in an oxygen-containing atmosphere at temperatures above 450 °C, to form fluorinated aromatic hydrocarbons. This reaction is simpler than the Sandmeyer reaction, but is only applicable for compounds that are stable enough to survive the high temperature.
Half mole of oxygen is used with 2 HF and Cu to make a mole of water and copper(II) fluoride.
Copper(II) fluoride can also be used to form fluorobenzene from benzene, HCl, and oxygen. Using a metal fluoride that can successfully oxidize with a methyl bond. The reaction is started by producing copper(II) fluoride with a charged metal reactor that contained copper oxide and then adding HF at high temperatures from there it is exposed to benzene and fluoroaromatics take place causing the formation of fluorotoluene or fluorobenzene or some other fluorinated benzene derivative. This enables fluoroaromatics to be done in large scale quantity without the waste disposals of the current fluorination processes.
Copper(II) fluoride can be synthesised from copper and fluorine at temperatures of 400 °C. It occurs as a direct reaction.
It loses fluorine in molten stage at temperatures above 950 °C.
The complex anions of CuF3−, CuF42− and CuF64− are formed if CuF2 is exposed to substances containing fluoride ions F−.
It is slightly soluble in water but it starts to decompose when in hot water, to form basic F− and Cu(OH) ions.
Copper(II) fluoride together has not been researched well on toxicity levels. However the toxicity affect of copper compounds and fluoride compounds individually.
...
Wikipedia
