Chlorite
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Chlorite
|
|
| Identifiers | |
| 14998-27-7 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.123.477 |
|
|
| Properties | |
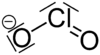
| ClO− 2 |
|
| Molar mass | 67.452 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
The chlorite ion, or chlorine dioxide anion, is ClO−
2. A chlorite (compound) is a compound that contains this group, with chlorine in oxidation state +3. Chlorites are also known as salts of chlorous acid.
The free acid, chlorous acid HClO2, is the least stable oxoacid of chlorine and has only been observed as an aqueous solution at low concentrations. Since it cannot be concentrated, it is not a commercial product. The alkali metal and alkaline earth metal compounds are all colorless or pale yellow, with sodium chlorite (NaClO2) being the only commercially important chlorite. Heavy metals chlorites (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and NH+
4) are unstable and decompose explosively with heat or shock.
Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. First, the explosively unstable gas chlorine dioxide, ClO2 is produced by reducing sodium chlorate in a strong acid solution with a suitable reducing agent (for example, sodium chloride, sulfur dioxide, or hydrochloric acid).
The chlorite ion adopts a bent molecular geometry, due to the effects of the lone pairs on the chlorine atom, with an O–Cl–O bond angle of 111° and Cl–O bond lengths of 156 pm. When compared to other chlorine oxyanions (on the basis of standard half cell potentials) chlorite is one strongest oxidizing agents in the series.
...
Wikipedia
