Cabozantinib
 |
|
| Clinical data | |
|---|---|
| Trade names | Cabometyx, Cometriq |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ≥99.7% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Biological half-life | 55 hours |
| Excretion | Faeces (54%), urine (27%) |
| Identifiers | |
|
|
| Synonyms | XL184, BMS907351 |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.221.147 |
| Chemical and physical data | |
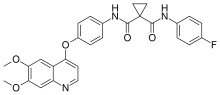
| Formula | C28H24FN3O5 |
| Molar mass | 501.51 g mol |
| 3D model (Jmol) | |
|
|
|
|
Cabozantinib, marketed under the trade name Cabometyx among others, is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and has been shown to reduce tumor growth, metastasis, and angiogenesis. It was discovered and developed by Exelixis Inc.
Cabozantinib was granted orphan drug status by the U.S. Food and Drug Administration (FDA) in January 2011. Cabozantinib is approved by the U.S. FDA for medullary thyroid cancer. and advanced renal cell carcinoma in people who have received prior anti-angiogenic therapy. It is currently undergoing clinical trials for the treatment of prostate, bladder, ovarian, brain, melanoma, breast, non-small cell lung, pancreatic, and hepatocellular cancers.
Cabozantinib will be distributed in Europe by the French pharmaceutical company Ipsen after a collaboration was reached with Exelixis in March 2016.
In October 2011, cabozantinib met its primary endpoint in a phase 3 clinical trial (EXAM) conducted by Exelixis investigating its effect on progression-free survival in medullary thyroid cancer. A new drug application was submitted in the first half of 2012, and on November 29, 2012 cabozantinib in its capsule formulation was granted marketing approval by the U.S. FDA under the name Cometriq for treating patients with medullary thyroid cancer.
Approval for its tablet formulation was granted for treating people with kidney cancer on April 25th, 2016.
Grapefruit and grapefruit juice should be avoided as they may increase the concentration of the drug in the blood. It is not yet known if cabozantinib is safe and effective in children.
It is undergoing clinical trials for the treatment of prostate, ovarian, brain, melanoma, breast, non-small cell lung, hepatocellular and kidney cancers.
...
Wikipedia
