Buspar
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ˈbjuːspᵻroʊn/ (BEW-spi-rohn) |
| Trade names | Buspar |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688005 |
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 3.9% |
| Protein binding | 86–95% |
| Metabolism | Hepatic (via CYP3A4) |
| Biological half-life | 2.5 hours |
| Excretion |
Urine: 29–63% Feces: 18–38% |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.048.232 |
| Chemical and physical data | |
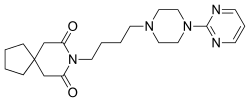
| Formula | C21H31N5O2 |
| Molar mass | 385.50314 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Buspirone, brand name Buspar, is an anxiolytic drug that is primarily used to treat generalized anxiety disorder (GAD). It is also commonly used to augment antidepressants in the treatment of depression. Unlike most anxiolytics, the pharmacology of buspirone is not related to that of benzodiazepines, barbiturates, or carbamates (it is not a GABA receptor agonist), and so buspirone does not carry the risk of physical dependence and withdrawal symptoms for which those drug classes are known. Buspirone is not considered to be a drug-of-abuse, is safer in overdose than traditional anxiolytics, and is significantly less impairing at therapeutic doses.
Buspirone is approved in the United States by the Food and Drug Administration (FDA) for the short- or long-term treatment of anxiety disorders or can also be used for the short-term relief of the symptoms of anxiety. Likewise in Australia, buspirone is licensed for the treatment of anxiety disorders. In the United Kingdom, buspirone is indicated only for the short-term treatment of anxiety.
Buspirone has no immediate anxiolytic effects, and hence has a delayed onset of action; its full clinical effectiveness may require 2 to 4 weeks to manifest. The drug has been shown to be similarly effective in the treatment of GAD to benzodiazepines including diazepam, alprazolam, lorazepam, and clorazepate. Buspirone is not known to be effective in the treatment of other anxiety disorders besides GAD, although there is some limited evidence that it may be useful in the treatment of social phobia as an adjunct to selective serotonin reuptake inhibitors (SSRIs). Buspirone allows fast-acting anxiety medications such as benzodiazepines to be effective at a much lower dose which greatly reduces the physical and mental side-effects of their use, making them feasible options for immediately treating anxiety when full cognitive function is required. Its lack of GABA-ergic activity also makes it a much safer option than traditional anxiolytics when used along with pain and seizure medications, and it is a first-line anxiety treatment for patients with chronic pain complaints.
...
Wikipedia
