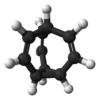
Bullvalene
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Tricyclo[3.3.2.02,8]deca-3,6,9-triene
|
|||
| Other names
Bullvalen
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C10H10 | |||
| Molar mass | 130.19 g/mol | ||
| Melting point | 96 °C (205 °F; 369 K) | ||
| Boiling point | decomposition at about 400 °C (752 °F; 673 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Bullvalene is a hydrocarbon with the chemical formula C10H10 with the unusual property that the chemical bonds making up the molecule are constantly rearranging as in fluxional molecules. For this reason bullvalene is extensively studied in organic chemistry.
The name bullvalene is derived from the nickname of one the scientists who predicted its properties in 1963 and the underlying concept of valence tautomerism,William "Bull" Doering. According to Klärner in 2011, the weekly seminars organised by Doering were secretly called "Bull sessions" by PhD students and postdocs and "were feared by those who were poorly prepared". The name was bestowed on the molecule, in 1961, by Doering's Yale graduate student, Maitland Jones. The name celebrates Bill Doering's well-known nickname and was chosen to rhyme with fulvalene, a molecule of great interest to the research group.
The bullvalene molecule is a cyclopropane platform with three vinylene arms conjoined at a methine group. As a fluxional molecule, bullvalene is subject to degenerate Cope rearrangements, with the result that all carbon atoms and hydrogen atoms appear equivalent on the NMR timescale. The number of possible valence tautomers of a bulvalene with ten distinguishable positions is 10!/3 = 1,209,600 not counting enantiomers.
The compound was first synthesised in 1963 by G. Schröder by photolysis of a dimer of cyclooctatetraene with expulsion of benzene.
The unusual dynamic properties of bullvalene have been examined by dynamic NMR spectroscopy. For example, the proton NMR spectrum changes from a sharp singlet at 4.2 ppm at higher temperatures to a complex pattern at lower temperatures. This pattern is consistent with an exchange process whose rate k is close to the frequency separation of the four contributing resonances.
...
Wikipedia