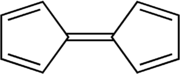
Fulvalene
 |
|
 |
|
| Names | |
|---|---|
| Other names
Bicyclopentyliden-2,4,2',4'-tetraene
1,1'-Bi[cylopentadienylidene] Pentafulvalene Bicyclopentadienylidene [5,5']Bicyclopentadienylidene |
|
| Identifiers | |
|
91-12-3 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:51994 |
| ChemSpider |
9083553 |
| PubChem | 10908294 |
|
|
|
|
| Properties | |
| C10H8 | |
| Molar mass | 128.17 g·mol−1 |
| Density | 1.129 g/ml |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2hsymmetry.
An earlier attempt at synthesis of fulvalene in 1951 by Pauson and Kealy resulted in the accidental discovery of ferrocene. Its synthesis was first reported in 1958 by E. A. Matzner at Yale University, working under William von Eggers Doering. In this method, dissertation only and never published, a cyclopentadienyl anion is coupled with iodine to the dihydrofulvalene which is then doubly deprotonated with n-butyllithium to the dianion and then oxidized with oxygen. Fulvalene was spectroscopically observed at 77 K from photolysis of diazocyclopentadiene (dimerization of two cyclopentadiene carbenes) with UV spectra matching those obtained by the Doering group. Final isolation of the compound came in 1986 via a method similar to that of Doering. The compound was found to be nonaromatic and extremely reactive above −50 °C through Diels-Alder dimerization.
...
Wikipedia
