Brompheniramine
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682545 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Biological half-life | 24.9 ± 9.3 hours |
| Excretion | Urine |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.001.507 |
| Chemical and physical data | |
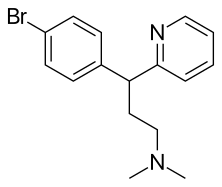
| Formula | C16H19BrN2 |
| Molar mass | 319.24 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Brompheniramine (Bromfed, Dimetapp, Bromfenex, Dimetane, BPN, Lodrane), commonly marketed as its salt brompheniramine maleate, is an antihistamine drug of the propylamine (alkylamine) class. It is readily available over the counter and is indicated for the treatment of the symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing. It is a first-generation antihistamine.
Brompheniramine is part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives and others including fluorpheniramine, chlorpheniramine, dexchlorpheniramine (Polaramine), triprolidine (Actifed), and iodopheniramine.
The halogenated alkylamine antihistamines all exhibit optical isomerism and brompheniramine products contain racemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.
Brompheniramine has antidepressant properties, inhibiting reuptake of the neurotransmitter serotonin and norepinephrine. Based on this knowledge, Arvid Carlsson and his colleagues, working at the Swedish company Astra AB, were able to derive the first marketed selective serotonin reuptake inhibitor, zimelidine, from brompheniramine.
...
Wikipedia
