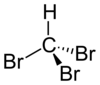
Bromoform
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Tribromomethane
|
|||
Other names
|
|||
| Identifiers | |||
|
75-25-2 |
|||
| 3D model (Jmol) | Interactive image | ||
| Abbreviations |
|
||
| 1731048 | |||
| ChEBI |
CHEBI:38682 |
||
| ChEMBL |
ChEMBL345248 |
||
| ChemSpider |
13838404 |
||
| DrugBank |
DB03054 |
||
| ECHA InfoCard | 100.000.777 | ||
| EC Number | 200-854-6 | ||
| 49500 | |||
| KEGG |
C14707 |
||
| MeSH | bromoform | ||
| PubChem | 5558 | ||
| RTECS number | PB5600000 | ||
| UNII |
TUT9J99IMU |
||
| UN number | 2515 | ||
|
|||
|
|||
| Properties | |||
| CHBr3 | |||
| Molar mass | 252.73 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 2.89 g mL−1 | ||
| Melting point | −4 to 16 °C; 25 to 61 °F; 269 to 289 K | ||
| Boiling point | 147 to 151 °C; 296 to 304 °F; 420 to 424 K | ||
| 3.2 g L−1 (at 30 °C) | |||
| log P | 2.435 | ||
| Vapor pressure | 670 Pa (at 20.0 °C) | ||
|
Henry's law
constant (kH) |
17 μmol Pa−1 kg−1 | ||
| Acidity (pKa) | 13.7 | ||
| -82.60·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.595 | ||
| Thermochemistry | |||
| 130.5 J K−1 mol−1 | |||
|
Std enthalpy of
formation (ΔfH |
6.1–12.7 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−549.1–−542.5 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
| GHS pictograms |
 
|
||
| GHS signal word | DANGER | ||
| H302, H315, H319, H331, H411 | |||
| P261, P273, P305+351+338, P311 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R22, R23, R36/38, R51/53 | ||
| S-phrases | (S1/2), S45, S63 | ||
| NFPA 704 | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
933.0 mg kg−1(oral, rat) | ||
|
LDLo (lowest published)
|
1400 mg/kg (mouse, oral) 1147 mg/kg (rat, oral) |
||
|
LC50 (median concentration)
|
1151 ppm (mammal) | ||
|
LCLo (lowest published)
|
4282 ppm (rat, 4 hr) 7000 ppm (dog, 1 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 0.5 ppm (5 mg/m3) [skin] | ||
|
REL (Recommended)
|
TWA 0.5 ppm (5 mg/m3) [skin] | ||
|
IDLH (Immediate danger)
|
850 ppm | ||
| Related compounds | |||
|
Related alkanes
|
|||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is a trihalomethane, and is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminum bromide. Currently its main use is as a laboratory reagent.
The molecule adopts tetrahedral molecular geometry with C3vsymmetry.
Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent.
Bromoform's high density makes it useful for separation of minerals by density. When two samples are mixed with bromoform and then allowed to settle, the top layer will contain minerals lighter than bromoform, and the bottom layer will contain heavier minerals. Slightly less dense minerals can be separated in the same way by mixing the bromoform with a small amount of a less dense and fully miscible solvent.
...
Wikipedia