Bendamustine
 |
|
| Clinical data | |
|---|---|
| Trade names | Treanda, Treakisym, Ribomustin, Levact, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608034 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
intravenous infusion |
| ATC code | L01AA09 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | NA (intravenous only) |
| Protein binding | 94–96% |
| Metabolism | Hydrolyzed to inactive metabolites. Two minor metabolites (M3 and M4) formed by CYP1A2 |
| Biological half-life | 40 min (bendamustine), 3 h (M3), 30 min (M4) |
| Excretion | ~50% urinary, ~25% fecal |
| Identifiers | |
|
|
| Synonyms | SDX-105 |
| CAS Number |
16506-27-7 |
| PubChem (CID) | 65628 |
| IUPHAR/BPS | 7478 |
| ChemSpider |
59069 |
| UNII |
9266D9P3PQ |
| ChEMBL |
CHEMBL487253 |
| ECHA InfoCard | 100.205.789 |
| Chemical and physical data | |
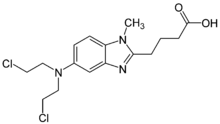
| Formula | C16H21Cl2N3O2 |
| Molar mass | 358.262 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Bendamustine, sold under the brand name Treanda among others, is a chemotherapy medication used in the treatment of chronic lymphocytic leukemia (CLL), multiple myeloma, and non-Hodgkin's lymphoma. It is given by injection into a vein.
Common side effects include low blood cell counts, fever, nausea, diarrhea, loss of appetite, cough, and rash. Other severe side effects include allergic reactions and increased risk of infection. Use in pregnancy is known to harm the baby. Bendamustine is in the alkylating agents family of medication. It works by interfering with the function of DNA and RNA.
Bendamustine was approved for medical use in the United States in 2008. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The cost in the United Kingdom for the NHS is 275.81 pounds per 100 mg vial. It was originally made from nitrogen mustard.
Bendamustine has been used both as sole therapy and in combination with other agents including etoposide, fludarabine, mitoxantrone, methotrexate, prednisone, rituximab, vincristine and 90Y-ibritumomab tiuxetan.
...
Wikipedia
