Barium iodide
 |
|
| Names | |
|---|---|
|
IUPAC name
Barium iodide
|
|
| Other names
Barium iodide, anhydrous
|
|
| Identifiers | |
|
13718-50-8 (anhydrous) 7787-33-9 (dihydrate) 13477-15-1 (hexahydrate) |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
75507 |
| ECHA InfoCard | 100.033.873 |
| EC Number | 237-276-9 |
| PubChem | 83684 |
|
|
|
|
| Properties | |
| BaI2 (anhydrous) BaI2·2H2O (dihydrate) |
|
| Molar mass | 391.136 g/mol (anhydrous) 427.167 g/mol (dihydrate) |
| Appearance | White orthorhombic crystals (anhydrous) colorless crystals (dihydrate) |
| Odor | odorless |
| Density | 5.15 g/cm3 (anhydrous) 4.916 g/cm3 (dihydrate) |
| Melting point | 711 °C (1,312 °F; 984 K) (anhydrous) decomposes at 740 °C (dihydrate) |
| 166.7 g/100 mL (0 °C) 221 g/100 mL (20 °C) 246.6 g/100 mL (70 °C) |
|
| Solubility | soluble in ethanol, acetone |
| -124.0·10−6 cm3/mol | |
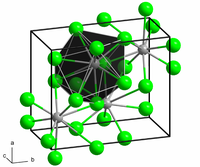
| Structure | |
| Orthorhombic, oP12, SpaceGroup = Pnma, No. 62 | |
| Thermochemistry | |
|
Std enthalpy of
formation (ΔfH |
-602.1 kJ·mol−1 |
| Hazards | |
| Main hazards | toxic |
| Related compounds | |
|
Other anions
|
barium fluoride barium chloride barium bromide |
|
Other cations
|
beryllium iodide magnesium iodide calcium iodide strontium iodide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Barium iodide is an inorganic compound with the formula BaI2. The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids. When heated, hydrated barium iodide converts to the anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
The structure of the anhydrous form resembles that of lead(II) chloride with each Ba center bound to nine iodide ligands and has a crystalline packing structure that is quite similar to BaCl2.
Anhydrous BaI2 can be prepared by treating Ba metal with 1,2-diiodoethane in ether.
BaI2 reacts with alkyl potassium compounds to form organobarium compounds.
BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.
Like other soluble salts of barium, barium iodide is toxic.
...
Wikipedia
