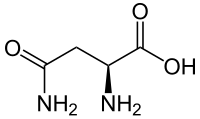
Asparagine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Asparagine
|
|
| Other names
2-Amino-3-carbamoylpropanoic acid
|
|
| Identifiers | |
|
70-47-3 |
|
| 3D model (Jmol) |
Interactive image Interactive image |
| ChEBI |
CHEBI:17196 |
| ChEMBL |
ChEMBL58832 |
| ChemSpider |
6031 |
| DrugBank |
DB03943 |
| ECHA InfoCard | 100.000.669 |
| EC Number | 200-735-9 |
| 4533 | |
| KEGG |
C00152 |
| PubChem | 236 |
| UNII |
7NG0A2TUHQ |
|
|
|
|
| Properties | |
| C4H8N2O3 | |
| Molar mass | 132.12 g·mol−1 |
| Appearance | white crystals |
| Density | 1.543 g/cm3 |
| Melting point | 234 °C (453 °F; 507 K) |
| Boiling point | 438 °C (820 °F; 711 K) |
| 2.94 g/100 mL | |
| Solubility | soluble in acids, bases, negligible in methanol, ethanol, ether, benzene |
| log P | −3.82 |
| Acidity (pKa) | 2.02 (carboxyl), 8.80 (amino) |
| -69.5·10−6 cm3/mol | |
| Structure | |
| orthorhomic | |
| Thermochemistry | |
|
Std enthalpy of
formation (ΔfH |
−789.4 kJ/mol |
| Hazards | |
| Safety data sheet |
See: data page Sigma-Alrich |
| NFPA 704 | |
| Flash point | 219 °C (426 °F; 492 K) |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Asparagine (abbreviated as Asn or N), encoded by the codons AAU and AAC, is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. A reaction between asparagine and reducing sugars or other source of carbonyls produces acrylamide in food when heated to sufficient temperature. These products occur in baked goods such as French fries, potato chips, and toasted bread.
Asparagine was first isolated in 1806 in a crystalline form by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant) from asparagus juice, in which it is abundant, hence the chosen name. It was the first amino acid to be isolated.
Three years later, in 1809, Pierre Jean Robiquet identified a substance from liquorice root with properties he qualified as very similar to those of asparagine, and that Plisson identified in 1828 as asparagine itself.
Since the asparagine side-chain can form hydrogen bond interactions with the peptide backbone, asparagine residues are often found near the beginning of alpha-helices as asx turns and asx motifs, and in similar turn motifs, or as amide rings, in beta sheets. Its role can be thought as "capping" the hydrogen bond interactions that would otherwise be satisfied by the polypeptide backbone. Glutamines, with an extra methylene group, have more conformational entropy and thus are less useful for capping.
...
Wikipedia

