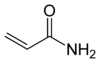
Acrylamide
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Prop-2-enamide
|
|||
| Other names
Acrylamide
Acrylic amide |
|||
| Identifiers | |||
|
79-06-1 |
|||
| 3D model (Jmol) |
Interactive image Interactive image |
||
| ChEBI |
CHEBI:28619 |
||
| ChEMBL |
ChEMBL348107 |
||
| ChemSpider |
6331 |
||
| ECHA InfoCard | 100.001.067 | ||
| 4553 | |||
| KEGG |
C01659 |
||
| PubChem | 6579 | ||
| UNII |
20R035KLCI |
||
|
|||
|
|||
| Properties | |||
| C3H5NO | |||
| Molar mass | 71.08 g·mol−1 | ||
| Appearance | white crystalline solid, no odor | ||
| Density | 1.13 g/cm3 | ||
| Melting point | 84.5 °C (184.1 °F; 357.6 K) | ||
| Boiling point | None (polymerization); decomposes at 175-300°C | ||
| 2.04 kg/L (25 °C) | |||
| Hazards | |||
| Main hazards | potential occupational carcinogen | ||
| Safety data sheet | ICSC 0091 | ||
| GHS pictograms |
 
|
||
| H301, H312, H315, H317, H319, H332, H340, H350, H361, H372 | |||
| P201, P280, P301+310, P305+351+338, P308+313 | |||
|
EU classification (DSD)
|
Toxic (T) Carc. Cat. 2 Muta. Cat. 2 Repr. Cat. 3 |
||
| R-phrases |
R45, R46, R20/21, R25, R36/38, R43, R48/23/24/25, R62 |
||
| S-phrases | S53, S45 | ||
| NFPA 704 | |||
| Flash point | 138 °C (280 °F; 411 K) | ||
| 424 °C (795 °F; 697 K) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
100-200 mg/kg (mammal, oral) 107 mg/kg (mouse, oral) 150 mg/kg (rabbit, oral) 150 mg/kg (guinea pig, oral) 124 mg/kg (rat, oral) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 0.3 mg/m3 [skin] | ||
|
REL (Recommended)
|
Ca TWA 0.03 mg/m3 [skin] | ||
|
IDLH (Immediate danger)
|
60 mg/m3 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Acrylamide (or acrylic amide) is a chemical compound with the chemical formula C3H5NO. Its IUPAC name is prop-2-enamide. It is a white odorless crystalline solid, soluble in water, ethanol, ether, and chloroform. Acrylamide decomposes in the presence of acids, bases, oxidizing agents, iron, and iron salts. It decomposes non-thermally to form ammonia, and thermal decomposition produces carbon monoxide, carbon dioxide, and oxides of nitrogen.
Acrylamide can be prepared by the hydrolysis of acrylonitrile by nitrile hydratase. In industry, most acrylamide is used to synthesize polyacrylamides, which find many uses as water-soluble thickeners. These include use in wastewater treatment, gel electrophoresis (SDS-PAGE), papermaking, ore processing, tertiary oil recovery, and the manufacture of permanent press fabrics. Some acrylamide is used in the manufacture of dyes and the manufacture of other monomers.
...
Wikipedia