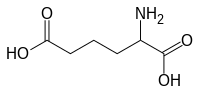
Alpha-Aminoadipic acid
 |
|
| Names | |
|---|---|
|
IUPAC name
2-aminohexanedioic acid
|
|
| Identifiers | |
|
542-32-5 |
|
| 3D model (Jmol) |
Interactive image Interactive image |
| ChEBI |
CHEBI:37024 |
| ChEMBL |
ChEMBL433238 |
| ChemSpider |
456 |
| MeSH | 2-Aminoadipic+Acid |
| PubChem | 469 |
|
|
|
|
| Properties | |
| C6H11NO4 | |
| Molar mass | 161.156 g/mol |
| Appearance | Crystalline |
| Density | 1.333 g/mL |
| Melting point | 196 °C (385 °F; 469 K) |
| Boiling point | 364 °C (687 °F; 637 K) |
| Hazards | |
| Main hazards | Irritant |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
The α-aminoadipate pathway is a biochemical pathway for the synthesis of the amino acid L-lysine. In the eukaryotes, this pathway is unique to the higher fungi (containing chitin in their cell walls) and the euglenids. It has also been reported from bacteria of the genus Thermus.
Homocitrate is initially synthesised from acetyl-CoA and 2-oxoglutarate by homocitrate synthase. This is then converted to homoaconitate by homoaconitase and then to homoisocitrate by homoisocitrate dehydrogenase. A nitrogen atom is added from glutamate by aminoadipate aminotransferase to form the α-aminoadipate from which this pathway gets its name. This is then reduced by aminoadipate reductase via an acyl-enzyme intermediate to a semialdehyde. Reaction with glutamate by one class of saccharopine dehydrogenase yields saccharopine which is then cleaved by a second saccharopine dehydrogenase to yield lysine and oxoglutarate.
α-Aminoadipic acid is an intermediate in the α-Aminoadipic acid pathway for the metabolism of lysine and saccharopine. It is synthesised from homoisocitrate by aminoadipate aminotransferase and reduced by aminoadipate reductase to form the semialdehyde.
...
Wikipedia
