Adrafinil
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Olmifon |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Metabolism | 75% (Liver) |
| Metabolites | Modafinil |
| Biological half-life | 1 hour (T1/2 is 12–15 hours for modafinil) |
| Excretion | Kidney |
| Identifiers | |
|
|
| Synonyms | CRL-40028 |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.058.440 |
| Chemical and physical data | |
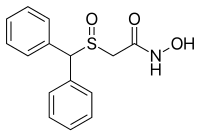
| Formula | C15H15NO3S |
| Molar mass | 289.351 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Adrafinil (INN) (brand name Olmifon) is a discontinued wakefulness-promoting agent (or eugeroic) that was formerly used in France to promote vigilance (alertness), attention, wakefulness, mood, and other parameters, particularly in the elderly. It was also used off-label by individuals who wished to avoid fatigue, such as night workers or others who needed to stay awake and alert for long periods of time. Additionally, "adrafinil is known to a larger nonscientific audience, where it is considered to be a nootropic agent."
Adrafinil is a prodrug; it is primarily metabolized in vivo to modafinil, resulting in very similar pharmacological effects. Unlike modafinil, however, it takes time for the metabolite to accumulate to active levels in the bloodstream. Effects usually are apparent within 45–60 minutes when taken orally on an empty stomach.
Adrafinil was marketed in France under the trade name Olmifon until September 2011 when it was voluntarily discontinued.
Because α1-adrenergic receptor antagonists were found to block effects of adrafinil and modafinil in animals, "most investigators assume[d] that adrafinil and modafinil both serve as α1-adrenergic receptor agonists." However, adrafinil and modafinil have not been found to bind to the α1-adrenergic receptor and they lack peripheral sympathomimetic side effects associated with activation of this receptor; hence, the evidence in support of this hypothesis is weak, and other mechanisms are probable. Modafinil was subsequently screened at a variety of targets in 2009 and was found to act as a weak, atypical blocker of the dopamine transporter (and hence as a dopamine reuptake inhibitor), and this action may explain some or all of its pharmacological effects. Relative to adrafinil, modafinil possesses greater specificity in its action, lacking or having a reduced incidence of many of the common side effects of the former (including stomach pain, skin irritation, anxiety, and elevated liver enzymes with prolonged use).
...
Wikipedia
