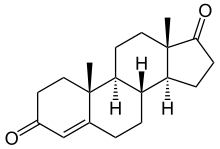
4-androstenedione
 |
|
 |
|
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Identifiers | |
|
|
| CAS Number |
63-05-8 |
| PubChem (CID) | 6128 |
| IUPHAR/BPS | 2860 |
| DrugBank |
DB01536 |
| ChemSpider |
5898 |
| UNII |
409J2J96VR |
| ChEBI |
CHEBI:16422 |
| ChEMBL |
CHEMBL274826 |
| ECHA InfoCard | 100.000.504 |
| Chemical and physical data | |
| Formula | C19H26O2 |
| Molar mass | 286.4 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 173–174 °C (343–345 °F) |
|
|
|
|
Δ4-Androstenedione (abbreviated as Δ4-dione), commonly referred to simply as androstenedione, and also known as androst-4-ene-3,17-dione, 4-androstene-3,17-dione or 17-ketotestosterone, is an endogenous androgen steroid hormone and intermediate in the biosynthesis of testosterone from dehydroepiandrosterone (DHEA). In turn, Δ4-dione is also a precursor of dihydrotestosterone (DHT), estrogens such as estradiol and estrone, and the neurosteroid 3α-androstanediol.
Δ4-Dione is the common precursor of the androgen and estrogen sex hormones.
Δ4-Dione can be biosynthesized in one of two ways. The primary pathway involves conversion of 17α-hydroxypregnenolone to DHEA by way of 17,20-lyase, with subsequent conversion of DHEA to Δ4-dione via the enzyme 3β-hydroxysteroid dehydrogenase. The secondary pathway involves conversion of 17α-hydroxyprogesterone, most often a precursor to cortisol, to Δ4-dione directly by way of 17,20-lyase. Thus, 17,20-lyase is required for the synthesis of Δ4-dione, whether immediately or one step removed.
...
Wikipedia
