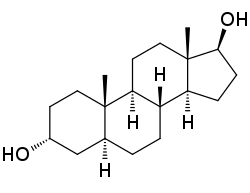
3α-Androstanediol
 |
|
| Names | |
|---|---|
|
IUPAC name
(3R,5S,8R,9S,10S,13S,14S,17S)-10,13-Dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,17-diol
|
|
| Other names
Hombreol
|
|
| Identifiers | |
| 1852-53-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 15039 |
| ECHA InfoCard | 100.015.862 |
| PubChem | 15818 |
|
|
|
|
| Properties | |
| C19H32O2 | |
| Molar mass | 292.46 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
3α-Androstanediol (often abbreviated as 3α-diol), also known as 5α-androstane-3α,17β-diol, is an endogenous inhibitory androstane neurosteroid and weak androgen, and a major metabolite of dihydrotestosterone (DHT). As a neurosteroid, it acts as a potent positive allosteric modulator of the GABAA receptor, and has been found to have rewarding,anxiolytic,pro-sexual, and anticonvulsant effects. As androgens such as testosterone and DHT are known to have many of the same effects as 3α-diol and are converted into it in vivo, it is thought that this compound may in part be responsible for said effects. Relative to its isomer 3β-androstanediol, which is a potent estrogen, 3α-androstanediol has substantially lower, though still significant affinity for the estrogen receptors, with a several-fold preference for ERβ over ERα. It has approximately 0.07% and 0.3% of the affinity of estradiol at the ERα and ERβ, respectively.
An orally active synthetic analogue of 3α-androstanediol, 17α-ethynyl-5α-androstane-3α,17β-diol (HE-3235, Apoptone), was formerly under investigation for the treatment of prostate cancer and breast cancer.
...
Wikipedia
