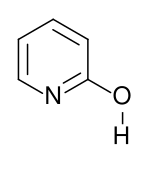
2-hydroxypyridine
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Pyridin-2(1H)-one
|
|||
| Other names
2(1H)-Pyridinone
2(1H)-Pyridone 1H-Pyridine-2-one 2-Pyridone 1,2-Dihydro-2-oxopyridine 1H-2-Pyridone 2-Oxopyridone 2-Pyridinol 2-Hydroxypyridine |
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.019 | ||
| RTECS number | UV1144050 | ||
|
|||
|
|||
| Properties | |||
| C5H5NO | |||
| Molar mass | 95.10 g·mol−1 | ||
| Appearance | Colourless crystalline solid | ||
| Density | 1.39 g/cm³ | ||
| Melting point | 107.8 °C (226.0 °F; 380.9 K) | ||
| Boiling point | 280 °C (536 °F; 553 K) decomp. | ||
| Solubility in other solvents | Soluble in water, methanol, acetone |
||
| Acidity (pKa) | 11.65 | ||
| UV-vis (λmax) | 293 nm (ε 5900, H2O soln) | ||
| Structure | |||
| Orthorhombic | |||
| planar | |||
| 4.26 D | |||
| Hazards | |||
| Main hazards | irritating | ||
| Safety data sheet | See: data page | ||
| R-phrases (outdated) | R36 R37 R38 | ||
| S-phrases (outdated) | S26 S37/39 | ||
| NFPA 704 | |||
| Flash point | 210 °C (410 °F; 483 K) | ||
| Related compounds | |||
|
Other anions
|
2-Pyridinolate | ||
|
Other cations
|
2-Hydroxypyridinium-ion | ||
|
Related functional groups
|
alcohol, lactam, lactim, pyridine, ketone |
||
|
Related compounds
|
pyridine, thymine, cytosine, uracil, benzene |
||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2-Pyridone is an organic compound with the formula C
5H
4NH(O). It is a colourless solid. It is well known to form hydrogen bonded dimers and it is also a classic case of a compound that exists as tautomers.
The most prominent feature of 2-pyridone is the amide group; a nitrogen with a hydrogen bound to it and a keto group next to it. In peptides, amino acids are linked by this pattern, a feature responsible for some remarkable physical and chemical properties. In this and similar molecules, the hydrogen bound to the nitrogen is suitable to form strong hydrogen bonds to other nitrogen- and oxygen-containing species.
The proton attached to the nitrogen can also move to the oxygen to give the second tautomer form, 2-hydroxypyridine. This lactam lactim tautomerism can also be exhibited in many related compounds.
The predominant solid state form is 2-pyridone. This has been confirmed by X-ray crystallography which shows that the hydrogen in solid state is closer to the nitrogen than to the oxygen (because of the low electron density at the hydrogen the exact positioning is difficult), and IR-spectroscopy, which shows that the C=O longitudinal frequency is present whilst the O-H frequencies are absent.
The determination of which of the two tautomeric forms is present in solution has been the subject of many publications. The energy difference appears to be very small and is dependent on the polarity of the solvent. Non-polar solvents favour the formation of 2-hydroxypyridine whereas polar solvents such as alcohols and water favour the formation of 2-pyridone.
...
Wikipedia