2-Naphthol
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Naphthalen-2-ol
|
|
| Other names
2-Hydroxynaphthalene; 2-Naphthalenol; beta-Naphthol; Naphth-2-ol
|
|
| Identifiers | |
|
135-19-3 |
|
| 3D model (Jmol) |
Interactive image Interactive image |
| ChEBI |
CHEBI:10432 |
| ChEMBL |
ChEMBL14126 |
| ChemSpider |
8341 |
| ECHA InfoCard | 100.004.712 |
| KEGG |
C11713 |
| PubChem | 8663 |
| UNII |
P2Z71CIK5H |
|
|
|
|
| Properties | |
| C10H8O | |
| Molar mass | 144.17 g·mol−1 |
| Appearance | Colorless crystalline solid |
| Density | 1.280 g/cm3 |
| Melting point | 121 to 123 °C (250 to 253 °F; 394 to 396 K) |
| Boiling point | 285 °C (545 °F; 558 K) |
| 0.74 g/L | |
| Acidity (pKa) | 9.51 |
| -98.25·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Harmful when inhaled or swallowed; dangerous to environment, esp. aquatic organisms. |
| R-phrases | R20 R22 R50 |
| S-phrases | S24 S25 S61 |
| NFPA 704 | |
| Flash point | 161 °C (322 °F) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
Traditionally, 2-naphthol is produced by a two-step process that begins with the sulfonation of naphthalene in sulfuric acid:
The sulfonic acid group is then cleaved in molten sodium hydroxide:
Neutralization of the product with acid gives 2-naphthol.
2-Naphthol can also be produced by a method analogous to the cumene process.
The Sudan dyes are popular dyes noted for being soluble in organic solvents. Several of the Sudan dyes are derived from 2-naphthol by coupling with diazonium salts. Sudan dyes I -IV and Sudan Red G consist of arylazo-substituted naphthols.
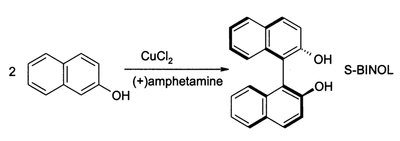
They can be also used in the production of dyes and in organic synthesis. For example, 2-naphthol reacts to form BINOL.
...
Wikipedia