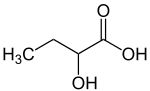
2-Hydroxybutyric acid
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
2-Hydroxybutanoic acid
|
|
| Other names
alpha-hydroxybutyrate
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.009.079 |
| MeSH | 2-hydroxybutyric+acid |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C4H8O3 | |
| Molar mass | 104.11 g·mol−1 |
| Related compounds | |
|
Other anions
|
hydroxybutyrate |
|
Related carboxylic acids
|
propionic acid lactic acid 3-hydroxypropionic acid malonic acid butyric acid hydroxypentanoic acid |
|
Related compounds
|
erythrose threose 1,2-butanediol 1,3-butanediol 2,3-butanediol 1,4-butanediol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
2-Hydroxybutyric acid, also known as alpha-hydroxybutyrate and α-hydroxybutyrate, is a hydroxybutyric acid with the hydroxyl group on the carbon adjacent to the carboxyl. It is a chiral compound having two enantiomers, D-2-hydroxybutyric acid and L-2-hydroxybutyric acid.
(R)-2-hydroxybutyric acid
(S)-2-hydroxybutyric acid
2-Hydroxybutyrate, the conjugate base of 2-hydroxybutyric acid, is produced in mammalian tissues (principally hepatic) that catabolize L-threonine or synthesize glutathione. Oxidative stress or detoxification demands can dramatically increase the rate of hepatic glutathione synthesis. Under such metabolic stress conditions, supplies of L-cysteine for glutathione synthesis become limiting, so homocysteine is diverted from the transmethylation pathway forming methionine into the transsulfuration pathway forming cystathionine. 2-Hydroxybutyrate is released as a byproduct when cystathionine is cleaved to cysteine that is incorporated into glutathione. Chronic shifts in the rate of glutathione synthesis may be reflected by urinary excretion of 2-hydroxybutyrate.
α-hydroxybutyrate may be useful as an early indicator of insulin resistance in non-diabetic subjects. Moreover, elevated serum α-hydroxybutyrate predicts worsening glucose tolerance.
...
Wikipedia
