Erythrose
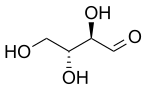
D-Erythrose
|
|

L-Erythrose
|
|
| Names | |
|---|---|
|
IUPAC names
(2R,3R)-2,3,4-Trihydroxybutanal (D)
(2S,3S)-2,3,4-Trihydroxybutanal (L) |
|
| Identifiers | |
|
583-50-6 (D) 533-49-3 (L) |
|
| 3D model (Jmol) | (D): Interactive image (L): Interactive image |
| ChEBI |
CHEBI:27904 |
| ChemSpider |
84990 (D) |
| ECHA InfoCard | 100.008.643 |
| PubChem | 94176 (D) |
|
|
|
|
| Properties | |
| C4H8O4 | |
| Molar mass | 120.10 g·mol−1 |
| Appearance | Light yellow syrup |
| Very soluble | |
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Erythrose is a tetrose carbohydrate with the chemical formula C4H8O4. It has one aldehyde group, and so is part of the aldose family. The natural isomer is D-erythrose.
Erythrose was first isolated in 1849 from rhubarb by the French pharmacist Louis Feux Joseph Garot (1798-1869), and was named as such because of its red hue in the presence of alkali metals (ἐρυθρός, "red").
Erythrose 4-phosphate is an intermediate in the pentose phosphate pathway and the Calvin cycle.
Oxidative bacteria can be made to use erythrose as its sole energy source.
...
Wikipedia

