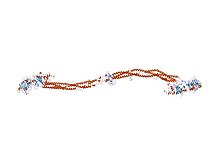
Fibrinogen
Fibrinogen (factor I) is a glycoprotein in vertebrates that helps in the formation of blood clots. It consists of a linear array of three nodules held together by a very thin thread which is estimated to have a diameter between 8 and 15 Angstrom (Å). The two end nodules are alike but the center one is slightly smaller. Measurements of shadow lengths indicate that nodule diameters are in the range 50 to 70 Å. The length of the dried molecule is 475 ± 25 Å.
The fibrinogen molecule is a soluble, large, and complex 340 kDa plasma glycoprotein, that is converted by thrombin into fibrin during blood clot formation. It has a rod-like shape with dimensions of 9 × 47.5 × 6 nm and it shows a negative net charge at physiological pH (IP at pH 5.2). Fibrinogen is synthesized in the liver by the . The concentration of fibrinogen in the blood plasma is 200–400 mg/dL (normally measured using the Clauss method).
During normal blood coagulation, a coagulation cascade activates the zymogen prothrombin by converting it into the serine protease thrombin. Thrombin then converts the soluble fibrinogen into insoluble fibrin strands. These strands are then cross-linked by factor XIII to form a blood clot. FXIIIa stabilizes fibrin further by incorporation of the fibrinolysis inhibitors alpha-2-antiplasmin and TAFI (thrombin activatable fibrinolysis inhibitor, procarboxypeptidase B), and binding to several adhesive proteins of various cells. Both the activation of factor XIII by thrombin and plasminogen activator (t-PA) are catalyzed by fibrin. Fibrin specifically binds the activated coagulation factors factor Xa and thrombin and entraps them in the network of fibers, thus functioning as a temporary inhibitor of these enzymes, which stay active and can be released during fibrinolysis. Research from 2011 has shown that fibrin plays a key role in the inflammatory response and development of rheumatoid arthritis.
...
Wikipedia