Zinc fluoride
 |
|
| Names | |
|---|---|
| Other names
Zinc difluoride
|
|
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.092 |
|
PubChem CID
|
|
| RTECS number | ZH3200000 |
|
|
|
|
| Properties | |
| ZnF2 | |
| Molar mass | 103.406 g/mol (anhydrous) 175.45 g/mol (tetrahydrate) |
| Appearance | white needles hygroscopic |
| Density | 4.95 g/cm3 (anhydrous) 2.30 g/cm3 (tetrahydrate) |
| Melting point | 872 °C (1,602 °F; 1,145 K) (anhydrous) 100 °C, decomposes (tetrahydrate) |
| Boiling point | 1,500 °C (2,730 °F; 1,770 K) (anhydrous) |
| .000052 g/100 mL (anhydrous) 1.52 g/100 mL, 20 °C (tetrahydrate) |
|
| Solubility | sparingly soluble in HCl, HNO3, ammonia |
| −38.2·10−6 cm3/mol | |
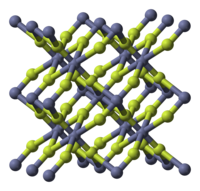
| Structure | |
| tetragonal (anhydrous), tP6 | |
| P42/mnm, No. 136 | |
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Zinc fluoride (ZnF2) is an inorganic chemical compound. It is encountered as the anydrous form and also as the tetrahydrate, ZnF2 · 4H2O (rhombohedral crystal structure). It has a high melting point and has the rutile structure containing 6 coordinate zinc, which suggests appreciable ionic character in its chemical bonding. Unlike the other zinc halides, ZnCl2, ZnBr2 and ZnI2, it is not very soluble in water.
Zinc fluoride can be synthesized several ways.
Zinc fluoride can be hydrolysed by hot water to form the zinc hydroxyfluoride, Zn(OH)F.
...
Wikipedia

