Zelboraf
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɛməˈræfənɪb/ VEM-ə-RAF-ə-nib |
| Trade names | Zelboraf |
| Synonyms | PLX4032, RG7204, RO5185426 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.226.540 |
| Chemical and physical data | |
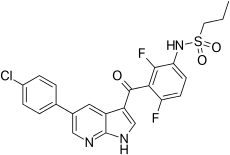
| Formula | C23H18ClF2N3O3S |
| Molar mass | 489.92 g/mol |
| 3D model (JSmol) | |
|
|
|
|
| Drug mechanism | |
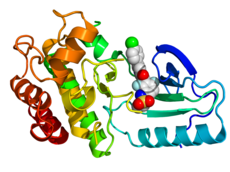
Crystallographic structure of B-Raf (rainbow colored, N-terminus = blue, C-terminus = red) complexed with vemurafenib (spheres, carbon = white, oxygen = red, nitrogen = blue, chlorine = green, fluorine = cyan, sulfur = yellow).
|
|
| Therapeutic use | melanoma |
|---|---|
| Biological target | BRAF |
| Mechanism of action | protein kinase inhibitor |
| External links | |
| ATC code | L01XE15 |
| PDB ligand id | 032: PDBe, RCSB PDB |
| LIGPLOT | 3og7 |
Vemurafenib (INN, marketed as Zelboraf) is a B-Raf enzyme inhibitor developed by Plexxikon (now part of Daiichi-Sankyo) and Genentech for the treatment of late-stage melanoma. The name "vemurafenib" comes from V600E mutated BRAF inhibition.
Vemurafenib received FDA approval for the treatment of late-stage melanoma on August 17, 2011, making it the first drug designed using fragment-based lead discovery to gain regulatory approval.
Vemurafenib later received Health Canada approval on February 15, 2012.
On February 20, 2012, the European Commission approved vemurafenib as a monotherapy for the treatment of adult patients with BRAF V600E mutation positive unresectable or metastatic melanoma, the most aggressive form of skin cancer.
Vemurafenib causes programmed cell death in melanoma cell lines. Vemurafenib interrupts the B-Raf/MEK step on the B-Raf/MEK/ERK pathway − if the B-Raf has the common V600E mutation.
Vemurafenib only works in melanoma patients whose cancer has a V600E BRAF mutation (that is, at amino acid position number 600 on the B-Raf protein, the normal valine is replaced by glutamic acid). About 60% of melanomas have this mutation. It also has efficacy against the rarer BRAF V600K mutation. Melanoma cells without these mutations are not inhibited by vemurafenib; the drug paradoxically stimulates normal BRAF and may promote tumor growth in such cases.
...
Wikipedia
