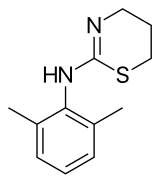
Xylazine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Rompun, Anased, Sedazine, Chanazine |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
oral, inhalation, or injection (intravenous, intramuscular, or subcutaneous) |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.028.093 |
| Chemical and physical data | |
| Formula | C12H16N2S |
| Molar mass | 220.33 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Xylazine is an analogue of clonidine and an agonist at the α2 class of adrenergic receptor. It is used for sedation, anesthesia, muscle relaxation, and analgesia in animals such as horses, cattle and other non-human mammals. Veterinarians also use xylazine as an emetic, especially in cats.
In veterinary anesthesia, xylazine is often used in combination with ketamine. It is sold under many brand names worldwide, most notably the Bayer brand name Rompun. It is also marketed as Anased, Sedazine, and Chanazine. The drug interactions vary with different animals.
It has become a drug of abuse, particularly in Puerto Rico, where it is diverted from stocks used by equine veterinarians and used as a cutting agent for heroin.
Xylazine was discovered as an antihypertensive agent in 1962 by Farbenfabriken Bayer in Leverkusen, Germany. Results from early human clinical studies confirmed that xylazine has several central nervous system depressant effects. Xylazine administration is used for sedation, anesthesia, muscle relaxation, and analgesia. It causes a significant reduction in blood pressure and heart rate in healthy volunteers. Due to hazardous side effects, including hypotension and bradycardia, xylazine was not approved by the Food and Drug Administration (FDA) for human use. As a result, xylazine's mechanism of action in humans remains unknown.
...
Wikipedia
