Xanax
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | Alprazolam /ælˈpræzəlæm/ or /ælˈpreɪzəlæm/, Xanax /ˈzænæks/ |
| Trade names | Xanax, Niravam |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684001 |
| Pregnancy category |
|
| Dependence liability |
Physical: Moderate Psychological: High |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Metabolism | Hepatic, via |
| Biological half-life |
Immediate release: 11.2 hours, Extended release: 10.7–15.8 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.044.849 |
| Chemical and physical data | |
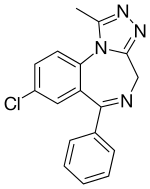
| Formula | C17H13ClN4 |
| Molar mass | 308.77 g·mol−1 |
| 3D model (Jmol) | |
|
|
|
|
Alprazolam, available under the trade name Xanax, is a potent, short-acting anxiolytic of the benzodiazepine class. It is commonly used for the treatment of anxiety disorders, especially of panic disorder, but also in the treatment of generalized anxiety disorder (GAD) or social anxiety disorder. It was the 12th most prescribed medicine in the United States in 2010. Alprazolam, like other benzodiazepines, binds to specific sites on the GABAA receptor. It possesses anxiolytic, sedative, hypnotic, skeletal muscle relaxant, anticonvulsant, amnestic, and antidepressant properties. Alprazolam is available for oral administration in compressed tablet (CT) and extended-release capsule (XR) formulations.
Peak benefits achieved for generalized anxiety disorder (GAD) may take up to a week.Tolerance to the anxiolytic/antipanic effects is controversial with some authoritative sources reporting the development of tolerance, and others reporting no development of tolerance; tolerance will, however, develop to the sedative-hypnotic effects within a couple of days.Withdrawal symptoms or rebound symptoms may occur after ceasing treatment abruptly following a few weeks or longer of steady dosing, and may necessitate a gradual dose reduction.
...
Wikipedia
