WSA Process
The wet sulfuric acid process (WSA process) is one of the key gas desulfurization processes on the market today. Since the Danish catalyst company Haldor Topsoe introduced and patented this technology in the late 1980s, it has been recognised as an efficient process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4), with simultaneous production of high pressure steam. The WSA process is applied in all industries where removal of sulfur is an issue.
The wet catalysis process is especially suited for processing one or more sulfur containing streams such as.:
The energy released by the above-mentioned reactions is used for steam production. Approximately 2–3 ton high-pressure steam per ton of acid produced.
Industries where WSA process plants are installed:
The acid gas coming from a Rectisol-, Selexol-, amine gas treating or similar installed after the gasifier contains H2S, COS and hydrocarbons in addition to CO2. These gases were previously often flared and vented to the atmosphere, but now the acid gas requires purification in order not to affect the environment with SO2 emission. Not only can the WSA process meet the demands of SO2 removal, the process also accepts a wide range of feed-gas compositions.
The WSA plant provides a high sulfur recovery and the heat recovered causes a substantial steam production. The heat recovery rate is high and the cooling water consumption low, resulting in superior cost performance of this process.
Example 1:
Example 2: A sulfur plant in China will be built in connection with an ammonia plant, producing 500 kilotons/annum of ammonia for fertilizer production
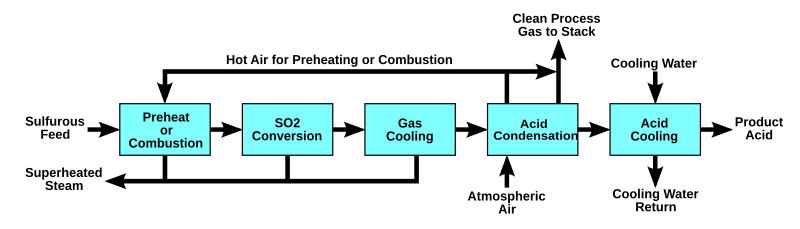
The WSA process can also be used for production of sulfuric acid from sulfur burning or for regeneration of the spent acid from e.g. alkylation plants. Wet catalysis processes differ from other contact sulfuric acid processes in that the feed gas contains excess moisture when it comes into contact with the catalyst. The sulfur trioxide formed by catalytic oxidation of the sulfur dioxide reacts instantly with the moisture to produce sulfuric acid in the vapour phase to an extent determined by the temperature. Liquid acid is subsequently formed by condensation of the sulfuric acid vapour and not by absorption of the sulfur trioxide in concentrated sulfuric acid, as is the case in contact processes based on dry gases.
...
Wikipedia