Vigabatrin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Sabril |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a610016 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 0% |
| Metabolism | not metabolized |
| Biological half-life | 5–8 hours in young adults, 12–13 hours in the elderly. |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.165.122 |
| Chemical and physical data | |
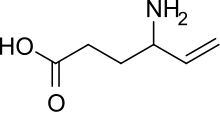
| Formula | C6H11NO2 |
| Molar mass | 129.157 g/mol |
| 3D model (Jmol) | |
| Melting point | 171 to 177 °C (340 to 351 °F) |
|
|
|
|
Vigabatrin, also known as gamma-vinyl-GABA, is an antiepileptic drug that inhibits the breakdown of γ-aminobutyric acid (GABA) by acting as a suicide inhibitor of GABA transaminase (GABA-T). It is a structural analog of GABA, but does not bind to GABA receptors. It is sold under the brand name Sabril.
In Canada, vigabatrin is approved for use as an adjunctive treatment (with other drugs) in treatment resistant epilepsy, complex partial seizures, secondary generalized seizures, and for monotherapy use in infantile spasms in West syndrome.
As of 2003, vigabatrin is approved in Mexico for the treatment of epilepsy that is not satisfactorily controlled by conventional therapy (adjunctive or monotherapy) or in recently diagnosed patients who have not tried other agents (monotherapy).
Vigabatrin is also indicated for monotherapy use in secondarily generalized tonic-clonic seizures, partial seizures, and in infantile spasms due to West syndrome.
On August 21, 2009, Lundbeck announced that the U.S. Food and Drug Administration had granted two New Drug Application approvals for vigabatrin. The drug is indicated as monotherapy for pediatric patients one month to two years of age with infantile spasms for whom the potential benefits outweigh the potential risk of vision loss, and as adjunctive (add-on) therapy for adult patients with refractory complex partial seizures (CPS) who have inadequately responded to several alternative treatments and for whom the potential benefits outweigh the risk of vision loss.
...
Wikipedia
