Vicriviroc
 |
|
| Names | |
|---|---|
|
IUPAC name
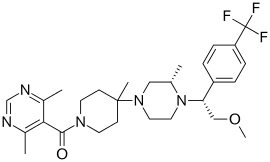
5-({4-[(3S)-4-{2-methoxy-1-[4-(trifluoromethyl)phenyl]ethyl}-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl}carbonyl)-4,6-dimethylpyrimidine
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| MeSH | Vicriviroc |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C28H38F3N5O2 | |
| Molar mass | 533.629 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.
The mechanisms of a number of available anti-HIV drugs prevent either viral reverse transcriptase enzyme or protease enzyme, allowing the virus to enter the cell before these drugs take effect. However, CCR5 inhibitors such as vicriviroc, as well as other entry inhibitors of HIV-1, inhibit the initial stages of the virus life cycle.
HIV binds to and fuses with the target T-cells or macrophages with the help of gp120 and gp41, the only two proteins that are currently known to be exhibited on the surface of the viral envelope. One molecule of each protein associates noncovalently with the other on the viral membrane, and three of these units aggregate to form the gp120/gp41 heterotrimer, which traps the gp41 in a conformationally metastable state.
Membrane fusion begins with the binding of gp120 to CD4, a glycoprotein which is expressed on the surface of the target cell. Upon binding, gp120 undergoes a conformational change, which causes the formation of the coreceptor binding site on gp120. All strains of HIV-1 use one of two coreceptors: CCR5 or CXCR4; coreceptor specificity will be described below. Once gp120 binds to the coreceptor, gp41 undergoes a conformational change that releases it from its once-metastable position. This change causes the hydrophobic N-terminus of the gp41 protein, also known as the fusion domain, to insert into the host cell membrane and anchor the virus into place. The insertion of gp41 into the target cell causes a subtle rearrangement in the gp41 protein that brings together two trimeric coiled coils, HR1 and HR2, to form a six-helix bundle. The bundle allows the viral and cellular membranes to approximate and eventually fuse together, leading to the release of the viral genome into the cytoplasm of the target cell.
...
Wikipedia
