Uranium(IV) oxide
 |
|
| Names | |
|---|---|
|
IUPAC names
Uranium dioxide
Uranium(IV) oxide |
|
| Other names
Urania
Uranous oxide |
|
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.014.273 |
| RTECS number | YR4705000 |
| Properties | |
| UO2 | |
| Molar mass | 270.03 g/mol |
| Appearance | black powder |
| Density | 10.97 g/cm3 |
| Melting point | 2,865 °C (5,189 °F; 3,138 K) |
| insoluble | |
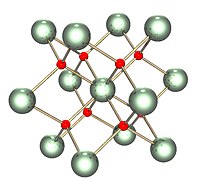
| Structure | |
| Fluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
|
a = 547.1 pm
|
|
| Tetrahedral (O2−); cubic (UIV) | |
| Thermochemistry | |
|
Std molar
entropy (S |
78 J·mol−1·K−1 |
|
Std enthalpy of
formation (ΔfH |
−1084 kJ·mol−1 |
| Hazards | |
| Safety data sheet | ICSC 1251 |
|
EU classification (DSD) (outdated)
|
Very toxic (T+) Dangerous for the environment (N) |
| R-phrases (outdated) | R26/28, R33, R51/53 |
| S-phrases (outdated) | (S1/2), S20/21, S45, S61 |
| Flash point | N/A |
| Related compounds | |
|
Triuranium octoxide Uranium trioxide |
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Uranium dioxide or uranium(IV) oxide (UO2), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used as MOX fuel. Prior to 1960, it was used as yellow and black color in ceramic glazes and glass.
Uranium dioxide is produced by reducing uranium trioxide with hydrogen.
This reaction plays an important part in the creation of nuclear fuel through nuclear reprocessing and uranium enrichment.
The solid is isostructural with (has the same structure as) fluorite (calcium fluoride), where each U is surrounded by eight O nearest neighbors in a cubic arrangement. In addition, the dioxides of cerium, plutonium and neptunium have the same structures. No other elemental dioxides have the fluorite structure. Upon melting, the measured average U-O coordination reduces from 8 in the crystalline solid (UO8 cubes), down to 6.7±0.5 (at 3270 K) in the melt. Models consistent with these measurements show the melt to consist mainly of UO6 and UO7 polyhedral units, where roughly 2/3 of the connections between polyhedra are corner sharing and 1/3 are edge sharing.
...
Wikipedia
