Uranium trioxide
 |
|
| Names | |
|---|---|
|
IUPAC names
Uranium trioxide
Uranium(VI) oxide |
|
| Other names
Uranyl oxide
Uranic oxide |
|
| Identifiers | |
|
1344-58-7 |
|
| ECHA InfoCard | 100.014.274 |
| Properties | |
| UO3 | |
| Molar mass | 286.29 g/mol |
| Appearance | yellow-orange powder |
| Density | 5.5–8.7 g/cm3 |
| Melting point | ~200–650 °C (decomposes) |
| Partially soluble | |
| Structure | |
| see text | |
| I41/amd (γ-UO3) | |
| Thermochemistry | |
|
Std molar
entropy (S |
99 J·mol−1·K−1 |
|
Std enthalpy of
formation (ΔfH |
−1230 kJ·mol−1 |
| Hazards | |
| Safety data sheet | External MSDS |
|
EU classification (DSD)
|
Very toxic (T+) Dangerous for the environment (N) |
| R-phrases | R26/28, R33, R51/53 |
| S-phrases | (S1/2), S20/21, S45, S61 |
| Flash point | Non-flammable |
| Related compounds | |
|
Uranium dioxide Triuranium octoxide |
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
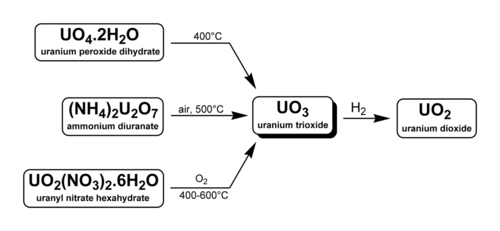
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.
There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium enrichment.
Uranium trioxide is shipped between processing facilities in the form of a gel, most often from mines to conversion plants. When used for conversion, all uranium oxides are often called reprocessed uranium(RepU).
Cameco Corporation, which operates at the world's largest uranium refinery at Blind River, Ontario, produces high-purity uranium trioxide.
It has been reported that the corrosion of uranium in a silica rich aqueous solution forms uranium dioxide, uranium trioxide, and coffinite. In pure water, schoepite (UO2)8O2(OH)12·12(H2O) is formed in the first week and then after four months studtite (UO2)O2·4(H2O) was produced. This alteration of uranium oxide also leads to the formation of metastudtite, a more stable uranyl peroxide, often found in the surface of spent nuclear fuel exposed to water. Reports on the corrosion of uranium metal have been published by the Royal Society.
...
Wikipedia