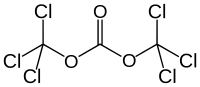
Triphosgene
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Bis(trichloromethyl) carbonate
|
|
| Other names
BTC
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.046.336 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C3Cl6O3 | |
| Molar mass | 296.748 g/mol |
| Appearance | white crystals |
| Density | 1.780 g/cm3 |
| Melting point | 80 °C (176 °F; 353 K) |
| Boiling point | 206 °C (403 °F; 479 K) |
| Reacts | |
| Hazards | |
| Safety data sheet | Fisher MSDS |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Triphosgene (bis(trichloromethyl) carbonate (BTC), C3Cl6O3) is a chemical compound that is used as a safer substitute for phosgene, because at room temperature it is a solid crystal, as opposed to phosgene which is a gas. Triphosgene crystals decompose above 200 °C
This compound is commercially available. It is prepared by exhaustive free radical chlorination of dimethyl carbonate:
Triphosgene can be easily recrystallized from boiling hexanes to yield pure white crystals.
Triphosgene is used as a reagent in organic synthesis and is a less hazardous substitute for phosgene for a variety of chemical transformations including to bond one carbonyl group to two alcohols, and to convert an amine group into isocyanate.
The toxicity of triphosgene is the same as phosgene since it decomposes to phosgene on heating and upon reaction with nucleophiles. Even trace moisture leads to formation of phosgene. Therefore, this reagent can be safely handled if one takes all the precautions as for phosgene.
...
Wikipedia
