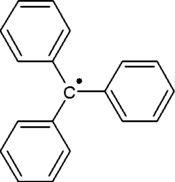
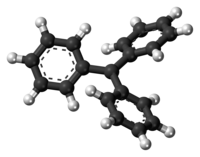
Triphenylmethyl radical
 |
|
 |
|
| Identifiers | |
|---|---|
|
3D model (JSmol)
|
|
| ChemSpider | |
|
|
|
|
| Properties | |
| C19H15 | |
| Molar mass | 243.33 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
The triphenylmethyl radical (often shorted to trityl radical) is a persistent radical and the first radical ever described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 (scheme 1) by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type dimer 3(3-triphenylmethyl-6-diphenylmethylidene-1,4-cyclohexadiene). In benzene the concentration of the radical is 2%.
Solutions containing the radical are yellow and when the temperature of the solution is raised the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following Le Chatelier's principle.
When exposed to air the radical rapidly oxidizes to the peroxide (Scheme 2) and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine to triphenylmethyl iodide.
The radical was discovered by Moses Gomberg in 1900 at the University of Michigan. He tried to prepare hexaphenylethane from triphenylmethyl chloride and zinc in benzene in a Wurtz reaction and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated.
...
Wikipedia
