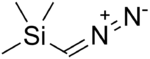
Trimethylsilyldiazomethane
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
(Diazomethyl)trimethylsilane
|
|
| Other names
Trimethylsilyldiazomethane
Diazo(trimethylsilyl)methane |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| 1902903 | |
| ChemSpider | |
| ECHA InfoCard | 100.131.243 |
| MeSH | Trimethylsilyldiazomethane |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C4H10N2Si | |
| Molar mass | 114.22 g·mol−1 |
| Appearance | greenish-yellow liquid |
| Boiling point | 96.0 °C (204.8 °F; 369.1 K) |
| Hazards | |
| Safety data sheet | External MSDS |
|
EU classification (DSD) (outdated)
|
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Trimethylsilyldiazomethane, (CH3)3SiCHN2, is a diazo compound widely used as a less-explosive replacement for diazomethane.
Trimethylsilyldiazomethane may be prepared from reacting (trimethylsilyl)methylmagnesium chloride with diphenyl phosphorazidate.
Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent. It is a less explosive alternative to diazomethane for the methylidenation of carboxylic acids. It also reacts with alcohols to give methyl ethers, where diazomethane may not.
It has also been employed widely in tandem with GC-MS for the analysis of various carboxylic compounds which are ubiquitous in nature. The fact that the reaction is rapid and occurs readily makes it attractive. However, it can form artifacts which complicate spectral interpretation. Such artifacts are usually the trimethylsilylmethyl esters, RCO2CH2SiMe3, formed when insufficient methanol is present. Acid-catalysed methanolysis is necessary to achieve near-quantitative yields of the desired methyl esters, RCO2Me.
The compound is a reagent in the Doyle-Kirmse reaction with allyl sulfides and allyl amines.
Inhalation of trimethylsilyldiazomethane is potentially fatal and has been implicated in the death of at least two chemists, a pharmaceutical worker in Windsor, Nova Scotia, Canada and one in New Jersey.
Although less likely to explode than diazomethane, trimethylsilyldiazomethane may be just as toxic. Inhalation of diazomethane is known to cause pulmonary edema; trimethylsilyldiazomethane is suspected to behave similarly.
When used as a reagent in organic synthesis to convert carboxylic acids to their methyl esters, trimethylsilyldiazomethane undergoes acid-catalysed methanolysis, forming diazomethane in situ. A similar hydrolysis reaction may take place when trimethylsilyldiazomethane comes into contact with water on the surface of a human lung.
...
Wikipedia
