Diazomethane
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Diazomethane
|
|
| Other names
Azimethylene,
Azomethylene, Diazirine |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.803 |
| KEGG | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| CH2N2 | |
| Molar mass | 42.04 g/mol |
| Appearance | Yellow gas |
| Odor | musty |
| Density | 1.4 (air=1) |
| Melting point | −145 °C (−229 °F; 128 K) |
| Boiling point | −23 °C (−9 °F; 250 K) |
| hydrolysis | |
| Structure | |
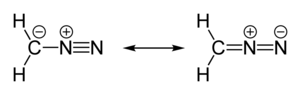
| linear C=N=N | |
| polar | |
| Hazards | |
| Main hazards | toxic and explosive |
| R-phrases | R12 R19 R22 R66 R67 |
| S-phrases | S9 S16 S29 S33 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LC50 (median concentration)
|
175 ppm (cat, 10 min) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 0.2 ppm (0.4 mg/m3) |
|
REL (Recommended)
|
TWA 0.2 ppm (0.4 mg/m3) |
|
IDLH (Immediate danger)
|
2 ppm |
| Related compounds | |
|
Related functional groups;
compounds |
R-N=N=N (azide), R-N=N-R (azo); R2CN2 R = Ph, tms, CF3 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions.
For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids into their methyl esters or into their homologues (see Arndt-Eistert synthesis). In the Büchner–Curtius–Schlotterbeck reaction diazomethane reacts with an aldehyde to form ketones.
When diazomethane is reacted with alcohols or phenols in presence of boron trifluoride (BF3), methyl ethers are obtained.
Diazomethane is also frequently used as a carbene source. It readily takes part in 1,3-dipolar cycloadditions.
Diazomethane is prepared by hydrolysis of an ethereal solution of an N-methyl nitrosamide with aqueous base. The traditional precursor is N-nitroso-N-methylurea, but this compound is itself somewhat unstable, and nowadays such compounds as N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) and N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) are preferred.
...
Wikipedia

