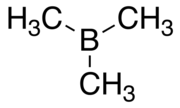
Trimethylborane
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Trimethylborane
|
|||
| Other names
trimethylborine, trimethylboron
|
|||
| Identifiers | |||
|
593-90-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
62201 |
||
| ECHA InfoCard | 100.008.926 | ||
| EC Number | 209-816-3 | ||
|
|||
|
|||
| Properties | |||
| C3H9B | |||
| Molar mass | 55.92 g/mol | ||
| Appearance | Colorless gas or liquid | ||
| Density | 0.625 g/cm3 at -100 °C | ||
| Melting point | −161.5 °C (−258.7 °F; 111.6 K) | ||
| Boiling point | −20.2 °C (−4.4 °F; 253.0 K) | ||
| slight, Highly reactive | |||
| Structure | |||
| Δ | |||
| Hazards | |||
| Main hazards | Spontaneously flammable in air; causes burns | ||
| R-phrases | R17 R34 | ||
| S-phrases | S7 S23 S26 S36/37/39 S43 S45 | ||
| −40 °C (−40 °F; 233 K) | |||
| Related compounds | |||
|
Related compounds
|
triethylborane Diborane methylborane |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl). Its melting point is -161.5 °C and its boiling point is -20.2 °C. Vapour pressure is given by log P = 6.1385+1.75 log T - 1393.3/T - 0.007735 T. T is temperature in Kelvin. CAS number is 593-90-8. Molecular weight is 55.914. The heat of vapourisation is 25.6 kJ/mol.
As a liquid it is colourless. The strongest line in the infrared spectrum is at 1330 cm−1 followed by lines at 3010 cm−1 and 1185 cm−1.
Trimethylborane was first made by Stock and Zeidler. Their method of preparation combined boron trichloride gas with dimethylzinc. Although the substance can be prepared using Grignard reagents the output is contaminated by unwanted products from the solvent. Trimethylborane can be made on a small scale with a 98% yield by reacting trimethylaluminium in hexane with boron tribromide in dibutyl ether as a solvent. Yet other methods are reacting tributyl borate with trimethylaluminium chloride, or potassium tetrafluoroborate with trimethylaluminium. Yet another method is to add boron trifluoride in ether to methyl magnesium iodide.
Trimethylborane spontaneously ignites in air if the concentration is high enough. It burns with a green flame producing soot. Slower oxidation with oxygen in a solvent or in the gas phase can produce dimethyltrioxadiboralane, which contains a ring of two boron and three oxygen atoms. However the major product is dimethylborylmethylperoxide, which rapidly decomposes to dimethoxymethylborane.
Trimethylborane is a strong Lewis acid. It reacts with water and chlorine at room temperature. It also reacts with grease but not with Teflon or glass. Trimethylborane can form an adduct with ammonia: (NH3):B(CH3)3.
...
Wikipedia