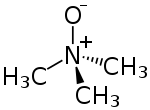
Trimethylamine N-oxide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
trimethylamine oxide
|
|
|
Preferred IUPAC name
N,N-dimethylmethanamine oxide
|
|
| Other names
trimethylamine oxide, TMAO, TMANO
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.341 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C3H9NO | |
| Molar mass | 75.11 |
| Appearance | colorless solid |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) (hydrate: 96 °C) |
| good | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Trimethylamine N-oxide (TMAO) is the organic compound in the class of amine oxides with the formula (CH3)3NO. This colorless solid is usually encountered as the dihydrate. It is a product of the oxidation of trimethylamine, a common metabolite in animals. It is an osmolyte found in saltwater fish, sharks, rays, molluscs, and crustaceans. It is a protein stabilizer that may serve to counteract urea, the major osmolyte of sharks, skates and rays. It is also higher in deep-sea fishes and crustaceans, where it may counteract the protein-destabilizing effects of pressure. TMAO decomposes to trimethylamine (TMA), which is the main odorant that is characteristic of degrading seafood.
TMAO can be synthesized from trimethylamine by treatment with hydrogen peroxide:
TMAO is biosynthesized from trimethylamine, which is derived from choline.
Trimethylaminuria is a rare defect in the production of the enzyme flavin containing monooxygenase 3 (FMO3). Those suffering from trimethylaminuria are unable to convert choline-derived trimethylamine into trimethylamine oxide. Trimethylamine then accumulates and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
Trimethylamine oxide is used in protein folding experiments to counteract the unfolding effects of urea.
In the organometallic chemistry reaction of nucleophilic abstraction, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
...
Wikipedia
