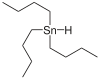
Tributyltin hydride
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
Tributylstannane
|
|||
| Identifiers | |||
|
688-73-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| 3587329 | |||
| ChEBI |
CHEBI:27086 |
||
| ChemSpider |
5734 |
||
| ECHA InfoCard | 100.010.642 | ||
| EC Number | 211-704-4 | ||
| 4258 | |||
| MeSH | Tributyltin | ||
| PubChem | 5948 | ||
|
|||
|
|||
| Properties | |||
| SnC 12H 28 |
|||
| Molar mass | 291.06 g mol−1 | ||
| Density | 1.082 g cm−3 | ||
| Boiling point | 80 °C (176 °F; 353 K) at 50 Pa | ||
| Slowly reacts | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.
The compound is produced by reduction of tributyltin oxide with polymethylhydrosiloxane (Bu = CH3CH2CH2CH2):
The hydride is a distillable liquid that is mildly sensitive to air, decomposing to (Bu3Sn)2O. Its IR spectrum exhibits a strong band at 1814 cm−1 for νSn-H.
It is a useful reagent in organic synthesis. Combined with azobisisobutyronitrile (AIBN) or by irradiation with light, tributyltin hydride converts organic halides (and related groups) to the corresponding hydrocarbon. This process occurs via a radical chain mechanism involving the radical Bu3Sn•. The radical abstracts a H• from another equivalent of tributyltin hydride, propagating the chain. Tributyltin hydride's utility as a H• donor can be attributed to its relatively weak bond strength (78 kcal/mol). Recently, transition metal hydrides have emerged as attractive alternatives to tin hydrides, as transition metal hydrides can be used catalytically and have more variety in bond strength.
...
Wikipedia