Trehalose
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
(2R,3S,4S,5R,6R)-2-(Hydroxymethyl)-6-[(2R,3R,4S,5S,6R)-3,4, 5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-3,4,5-triol
|
|
| Other names
α,α‐Trehalose; α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside
|
|
| Identifiers | |
|
99-20-7 (anhydrous) 6138-23-4 (dihydrate) |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:16551 |
| ChEMBL |
ChEMBL1236395 |
| ChemSpider |
7149 |
| ECHA InfoCard | 100.002.490 |
| PubChem | 7427 |
| UNII |
B8WCK70T7I |
|
|
|
|
| Properties | |
| C12H22O11 (anhydride) | |
| Molar mass | 342.296 g/mol (anhydrous) 378.33 g/mol (dihydrate) |
| Appearance | White orthorhombic crystals |
| Density | 1.58 g/cm3 at 24 °C |
| Melting point | 203 °C (397 °F; 476 K) (anhydrous) 97 °C (dihydrate) |
| 68.9 g per 100 g at 20 °C | |
| Solubility | soluble in ethanol, insoluble in diethyl ether and benzene |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
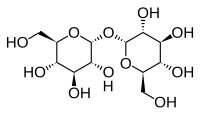
Trehalose, also known as mycose or tremalose, is a natural alpha-linked disaccharide formed by an α,α-1,1-glucoside bond between two α-glucose units. In 1832, H.A.L. Wiggers discovered trehalose in an ergot of rye, and in 1859 Marcellin Berthelot isolated it from trehala manna, a substance made by weevils, and named it trehalose. It can be synthesised by bacteria, fungi, plants, and invertebrate animals. It is implicated in anhydrobiosis — the ability of plants and animals to withstand prolonged periods of desiccation. It has high water retention capabilities, and is used in food and cosmetics. The sugar is thought to form a gel phase as cells dehydrate, which prevents disruption of internal cell organelles, by effectively splinting them in position. Rehydration then allows normal cellular activity to be resumed without the major, lethal damage that would normally follow a dehydration/rehydration cycle. Trehalose is not an antioxidant, because it is a non-reducing sugar and does not contain nucleophilic groups in its molecule. However, it was reported to have antioxidant effects.
Extracting trehalose was once a difficult and costly process, but circa the year 2000, the Hayashibara company (Okayama, Japan) confirmed an inexpensive extraction technology from starch for mass production.
Trehalose is used in a broad spectrum of applications.
Trehalose is a disaccharide formed by a 1,1-glucoside bond between two α-glucose units. Because trehalose is formed by the bonding of two reducing aldehyde groups, it has no capacity to participate in the Maillard reaction. There is an industrial process where trehalose is derived from corn starch. There are at least 3 biological pathways for trehalose biosynthesis.
...
Wikipedia
