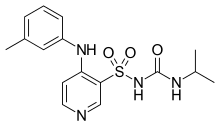
Torsemide
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601212 |
| Pregnancy category |
|
| Routes of administration |
Oral, IV |
| ATC code | C03CA04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80-90% |
| Protein binding | Highly bound (>99%). |
| Metabolism | Hepatic (80%) |
| Biological half-life | 3.5 hours; Cirrhosis: 7-8 hours |
| Identifiers | |
|
|
| CAS Number |
56211-40-6 |
| PubChem (CID) | 41781 |
| IUPHAR/BPS | 7312 |
| DrugBank |
DB00214 |
| ChemSpider |
38123 |
| UNII |
W31X2H97FB |
| KEGG |
D00382 |
| ChEBI |
CHEBI:9637 |
| ChEMBL |
CHEMBL1148 |
| ECHA InfoCard | 100.164.924 |
| Chemical and physical data | |
| Formula | C16H20N4O3S |
| Molar mass | 348.421 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Torasemide (rINN) or torsemide (USAN) is a pyridine-sulfonyl urea type loop diuretic mainly used in the management of edema associated with congestive heart failure. It is also used at low doses for the management of hypertension. It is marketed under the brand names Demadex, Diuver, and Examide.
Compared with other loop diuretics, torasemide has a more prolonged diuretic effect than equipotent doses of furosemide and relatively decreased potassium loss. No evidence of torasemide-induced ototoxicity has been demonstrated in humans.
Recent publications suggest improved outcomes when used for heart failure compared with furosemide.
...
Wikipedia
