Titanocene dichloride
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Dichloridobis(η5-cyclopentadienyl)titanium
|
|
| Other names
titanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV)
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.669 |
|
PubChem CID
|
|
| RTECS number | XR2050000 |
|
|
|
|
| Properties | |
| C10H10Cl2Ti | |
| Molar mass | 248.96 g/mol |
| Appearance | bright red solid |
| Density | 1.60 g/cm3, solid |
| Melting point | 289 °C (552 °F; 562 K) |
| sl. sol. with hydrolysis | |
| Structure | |
| Triclinic | |
| Dist. tetrahedral | |
| Hazards | |
| R-phrases | R37, R38 |
| S-phrases | S36 |
| NFPA 704 | |
| Related compounds | |
|
Related compounds
|
Ferrocene Zirconocene dichloride Hafnocene dichloride Vanadocene dichloride Niobocene dichloride Tantalocene dichloride Molybdocene dichloride Tungstenocene dichloride TiCl4 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
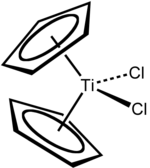
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. Cp2TiCl2 does not adopt the typical "sandwich" structure like ferrocene due to the 4 ligands around the metal centre, but rather takes on a distorted tetrahedral shape. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
Cp2TiCl2 continues to be prepared from titanium tetrachloride, in the same way as its original synthesis by Wilkinson and Birmingham:
The reaction is conducted in THF. Workup sometimes washing with hydrochloric acid to convert hydrolysis derivatives to the dichloride. Recrystallization from toluene forms acicular crystals.
Cp2TiCl2 can also be prepared by using freshly distilled cyclopentadiene:
This reaction is conducted under a nitrogen atmosphere and by using THF as solvent. The product is purified by soxhlet extraction using toluene as solvent.
The complex is pseudotetrahedral. Each of the two Cp rings are attached as η5 ligands. Viewing the Cp ligands as tridentate, the complex has a coordination number of 8.
...
Wikipedia

