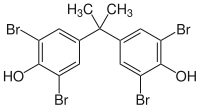
Tetrabromobisphenol A
 |
|
| Names | |
|---|---|
|
IUPAC name
2,2′,6,6′-Tetrabromo-4,4′-isopropylidenediphenol
|
|
| Other names
2,2′,6,6′-Tetrabromobisphenol A, 2,2-Bis(3,5-dibromo-4-hydroxyphenyl)propane, 4,4′-Isopropylidenebis(2,6-dibromophenol)
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| Abbreviations | TBBPA, TBBP-A, TBBA |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.125 |
| EC Number | 201-236-9 |
| KEGG | |
| MeSH | C443737 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C15H12Br4O2 | |
| Molar mass | 543.9 g·mol−1 |
| Density | 2,12 g·cm−3 (20 °C) |
| Melting point | 178 °C (352 °F; 451 K) |
| Boiling point | 250 °C (482 °F; 523 K) (decomposition) |
| insoluble | |
| Hazards | |
| Main hazards | N |
| R-phrases | R50/53 |
| S-phrases | S60 S61 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tetrabromobisphenol A (TBBPA) is a brominated flame retardant. The compound is a colorless solid, although commercial samples appear yellow. It is one of the most common fire retardants.
TBBPA is produced by the reaction of bromine with bisphenol A. Most commercial TBBPA products consist of a mixture that differ in the degree of bromination with the formula C15H16−xBrxO2 where x = 1 to 4. Its fire-retarding properties correlate with %Br.The annual consumption in Europe has been estimated as 6.200 tons in 2004.
TBBPA is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant polycarbonates by replacing some bisphenol A. A lower grade of TBBPA is used to prepare epoxy resins, used in printed circuit boards.
A study was published by the European Food Safety Authority (EFSA) in December 2011 on the exposure of TBBPA and its derivatives in food. The study, which examined at 344 food samples from the fish and other seafood food group, concluded that “current dietary exposure to TBBPA in the European Union does not raise a health concern.” EFSA also determined that “additional exposure, particularly of young children, to TBBPA from house dust is unlikely to raise a health concern”.
Some studies suggest that TBBPA may be an endocrine disruptor and immunotoxicant. As an endocrine disruptor, TBBPA may interfere with both estrogens and androgens. Further, TBBPA structurally mimics the thyroid hormone thyroxin (T4) and can bind more strongly to the transport protein transthyretin than T4 does, likely interfering with normal T4 activity. TBBPA likely also suppresses immune responses by inhibiting expression of CD25 receptors on T cells, preventing their activation, and by reducing natural killer cell activity.
...
Wikipedia
